

Research - African Journal of Food Science and Technology ( 2021) Volume 12, Issue 4
, DOI: 10.14303//ajfst.2021.025
Its food value and rich bioactives notwithstanding, processing avocado seeds (largely underutilized) into safe and acceptable 3G functional food snack has not been explored. The seeds(de-coated, blanched. dried, milled and sieved) were blended at different proportions with sweetened maize flour and subjected to extrusion (≈1000C, 100rpm) and hot air puffing (700C, 4hrs). Proximate, antioxidant properties and bioactive compounds of the resulting snack were evaluated. All were conducted in line with standard protocol. The snacks (mainly carbohydrate 47.3-61.8%) had low anti-nutrients with expansion ratios (2.8-3.1). Flavonoids (117.1-267.6mg/100g) and total antioxidant capacity (427.5-665.5 AAE) were significant (p≤0.05). A9, 12-Octadecadienoic acid was the predominant bioactive. Animal weight and malondialdehyde declined [110-102g; 10.18-3.58µm] in stressed avocado based snack-fed rats as against a rise in the control group. Overall organoleptically, 20% Avocado seedbased snack was most preferred. Safe and acceptable 3G avocado-based functional food snack with anti-stress and weight loss potentials is feasible, providing a novel outlet for the seed
Avocado seeds;3G Snack food; Antioxidative stress; Bioactive; Weight loss snacks
Avocado fruit aptly called the 'green gold' is one of the most common tropical fruits grown around the world with production put at over 6 million metric tons and still rising (FAO,2018). A significant component of the fruit is the seed - conical, round or ovoid, 2-2.5cm in length, and enclosed in an outer layer (seed coat) that is papery and brown. The seeds represent some 13-18% of the fruit not often utilized but discarded. This seeming waste may pose a serious ecological problem (Ortiz et al., 2004). It may however be of interest to the food and pharmaceutical industries since it contains an array of substances some of which are useful and physiologically active.
Indeed, the compositional profile of Avocado seeds is multifarious and quite beneficial. According to Ramos et al. (2004), the seeds mostly comprise of triterpenes, fatty acids, and phytosterols. They have also been found to exhibit antidiabetic effect, antihypertensive properties, and a cholesterol-lowering propensity (Imafidon and Amaechina 2010). The seed contains starch with good physicochemical and rheological properties (Chel-Guerrero et al., 2016) along with fibrous residues that can retain water and oil several times its weight (Barbosa-Martins et al., 2016). This makes it a potential raw material for many food systems. Interestingly, some of the aforementioned positive characteristics of the seed have been translated into a successful application as food additive/flour substitute in biscuits (Mahawan et al., 2015), natural yellow food colorant (Dabas et al., 2011) as well as anti-oxidant and microbicide in pork burgers and meat chops(Weiss et al., 2010) Undoubtedly, the use of underexploited materials such as Avocado seeds has far-reaching consequences for the health and overall wellbeing of those who regularly consume them as food. A significant contributor in this respect is regular metabolic processes and extraneous factors generating reactive oxygen species (ROS) (free radicals) in cellular fluids. Indeed, these species have been linked to oxidation in biological systems culminating in diseased states like cancer, neurological degeneration as well as rancidity in foods. Anti-dotes to these phenomena has been associated with antioxidants that offer some measure of protection on biomolecules. When consumed regularly (as in fruits) they have been shown to slow down cardiovascular degeneration and the aging process. This reduction is due to the presence of natural antioxidants such as phenolic acid, flavonoids, carotenoids abundant in fruits, vegetables, and their byproducts like avocado seeds (Rui, 2003).
In many African countries, Avocado seeds are traditionally consumed in soups and puddings because of their perceived antihypertensive benefits. Its bitter taste and brown color have however mitigated wide consumer acceptability (Anaka et al., 2009). There is therefore a need to explore possible processing methods of making the seeds more palatable and acceptable as with third-generation(3G) snack foods (eaten in-between meals), yet retaining most of its health-promoting properties. Notably, 3G snacks are semi-finished (half products) often in non-expanded pellets transformed to finished snacks only after expansion through exposure to either hot air, microwaving, or frying (Gandhi et al., 2016). Indeed, it remains one of the fastest-growing segments of the snack food subsector worldwide (Delgados- Nieblas, 2015; Watrous, 2019). It is the objective of this work therefore to evaluate the possibility of developing an acceptable 3G snack incorporating avocado seeds and ascertain their nutritional, antioxidative stress, and safety quality recent renewed interest in the use of several underexploited food materials such as avocado seeds beyond their nutritional value further underscores the need for this study.
Raw material Inputs and sourcing
Avocado fruits and Maize grains are the principal raw material inputs. The fruit was sourced from a local market in Bodija, Ibadan Nigeria while the maize grains were obtained from the Institute of Agriculture Research and Training (IAR&T), Moor Plantation, Ibadan.
Processing of Maize- Avocado based 3G Snack
Conversion of Avocado Seed into Flour:
The seeds were obtained from the pulp by gently slicing through the fruit and the outer coat was carefully removed. Hot water (70-80°C) blanching (1-2mins) of the seeds followed to reduce enzymatic browning, thereafter drained. It was next sliced into thin flakes, dried (cabinet drier, 60 °C for 48 h)(see supplementary files) milled and sieved (300 μm screen) to obtain a fine powder( packaged in polyethylene film and stored in a refrigerator).
Conversion of Maize grains into Flour:
The maize grains were first cleaned to remove the damaged kernel, stones, and other extraneous materials. They were next washed with potable water, dried (cabinet drier), milled (hammer mill) all in that order to obtain the flour. The resulting flour was sieved (300 μm screen) and stored in an airtight polythene bag.
Formulation and Processing of Maize- Avocado Seed based 3G Snack:
The formulation and extrusion of the Maize–avocado seed- based snack was based on the method of Balentic et al., (2018) with a slight adjustment. The snack was made by formulating composite blends of Maize and Avocado seed flour in selected ratios 90:10, 80:20, 70:30 respectively as well as 100% maize flour and 100% Avocado seed flour (Olatoye & Arueya,2018). Moisture content was adjusted to 14 g/100 g by adding distilled water and allowing the mixture to equilibrate for 4hrs at room temperature. The different proportions of the blends were mixed with other ingredients (see below). The mixture was subjected to a hot extrusion barrel at 110–115 °C with a Length to diameter (L/D) ratio of 12:1 and the screw speed of 100 rpm. The extruded snacks were then puffed at 70ºC for 4 h, cooled, and properly stored in airtight polyethylene. The other ingredients added were vanilla (1%), salt (2%), sugar (5%) (Figure 1).
Proximate Analysis:
The moisture, ash, protein, fat, and crude fiber content in the sample were determined in triplicates according to the methods of the Association of Official Analytical Chemists (AOAC, 2005). The total carbohydrate content was then estimated by difference.
Anti-nutritionalFactors
Determination of saponin content:
The Spectrophotometric method of Brunner (1984) was used for saponin analysis. One gram of finely ground sample was weighed into a 250 ml beaker and 100 ml Isobutyl alcohol was added. The mixture was shaken on a UDY shaker for 5 h to ensure uniform mixing. Samples of between 0-10 ppm standard saponin solutions were prepared from the saponin stock solution. The absorbances of the sample as well as standard saponin solutions were read after color development on a Spectronic 2lD Spectrophotometer at a wavelength of 380 nm. Percentage saponin was calculated using the formula:
Saponin (%) = Absorbance of sample x Average gradient x Dilution factor
Weight of sample x 10,000
Determination of tannin content:
The method of Talabi et al. (2016) was deployed for the determination of tannin contents with a slight adjustment. Aliquot amount (0.2 g) of finely ground sample was measured into a 50 ml beaker. About 20 ml of 50% methanol was added and covered with paraffin and placed in a water bath at 77-80°C for 1 h and stirred with a glass rod to prevent lumping. Standard Tannic Acid solutions of range 0-10 ppm were treated similarly as the 1 ml of the sample above. The absorbances of the Tannic acid standard solutions as well as samples were read after color development on a Spectronic 21D Spectrophotometer at a wavelength of 760 nm. Percentage tannin was calculated using the formula:
Tannin (%) = Absorbance of sample x Average gradient x Dilution factor
Weight of sample x 10,000
Determination of Phytate/Phytic acid:
Extraction and determination of phytate:
The extraction of phytate from the sample was carried out following a modified procedure of (Talabi et al., 2016). Some quantity (2.0 g) of the sample was extracted with 40 ml of 2.4% HCl (68.6 ml of 35% hydrochloric acid in a total volume of 1 liter of D2O) under constant shaking at room temperature (25°C) for 3 h. All extracts were then filtered using Whatman No. 1 filter paper. The content of phytate was determined using a spectrophotometric method, with an absorbance (A) wavelength at 640 nm (AOAC, 2005). The amount of phytic acid was calculated from the organic phosphorus by assuming that one molecule of phytic acid (containing six molecules of phosphorus (P)) was digested as shown in the equation below (AOAC, 2005):
Phytate mg/g sample
Mean = K" * A *20m
0:282 *1000
where A = absorbance;
‘‘K’’ = standard P (μg)/[A/volume (ml)];
Phytate = 28.2% P; 20 = extract volume (ml) of 1 g sample; 1000 = conversion from μg/g to mg/g. The results were reported in the percentage of phytate in 100 grams of sample.
Determination of Oxalate:
Oxalate was determined using the titration method described by AOAC (2005). Two grams of sample was suspended in a mixture of 190ml of distilled water in a 250ml volumetric flask. Some 6M HCl was added to 10ml of the suspension and heated for 1 hour at 100°C in a water bath. The mixture was cooled and made up to 250ml mark with distilled water before filtration. A duplicate portion of 125ml of the filtrate was measured into 250ml beakers. The filtrate was heated again to 900 C on a hot water bath and 10ml of 5% calcium chloride solution was added while being stirred constantly. After heating, it was centrifuged at full speed (2500 rpm) for 5minutes. The supernatant was decanted and dissolved in 10ml of 20% (v/v) H2SO4 solution and the total filtrate resulting from 2g of the sample was made up to 300ml.
Permanganate titration: 125ml of the filtrate was heated until near boiling and then titrated against 0.05M KMNO4 solution to a faint pink color.
Oxalic acid content was calculated using the formula, %Oxalic acid = T x (Vme) (Df) x 105 ME x Mf where, T = Titre of KMNO4 (ml), Vme = volume - mass equivalent (1ml of 0.05M MNO4 solution is equivalent to 0.0022g anhydrous oxalic acid), Df = the dilution factor (i.e 300ml) 125ml, ME = the molar equivalent of KMNO4 in oxalic acid (KMNO4 redox reaction is 5), Mf = the mass of the sample use.
Physical Properties of Maize- Avocado Seed based 3G snack food
Bulk Density
Loosed and packed bulk densities were determined according to the procedures of Arueya & Ugwu (2017). Some 10grams of the sample(W) were weighed into a 50ml measuring cylinder and the sample inside the cylinder was tapped several times for 10 minutes to eliminate spaces within the flour till a constant volume (V) was obtained.
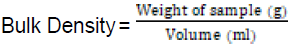
Swelling capacity and solubility power:
Following the method described by AOAC (2000), one gram of each sample was weighed into a conical flask, and 15ml of distilled water was added. The mixtures were shaken for 5 minutes and heated on a water bath for 40 minutes at 80 – 85°C with consistent stirring. The resulting solutions were then transferred into pre-weighed centrifuge tubes. Some 7.5ml of distilled water was added into each centrifuge tube and centrifuged at 2,200 rpm for 20 minutes. The resulting supernatant was decanted into pre-weighed cans and dried at 100°C. to a constant weight. These were then cooled in a desiccator and weighed.
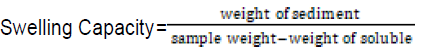
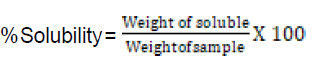
Expansion Ratio
The diameter of the snack food products was measured using calipers. The expansion ratio (ER) of the snack food sample was calculated by dividing their diameter (D) by the extruder die diameter (d2). Each value was an average of ten measurements.
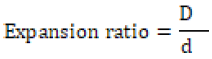
Antioxidant Potential
Total Phenol Content:
The Folin- Ciocalteu method was used for the analysis of the total phenol content of the samples with few adjustments (Arueya et al., 2017). One milliliter of appropriate dilutions (10–1000 μg) of the sample extract and 1ml Folin-Ciocalteu reagent were mixed. The resulting mixture after some three minutes was neutralized through the addition of 1ml of a saturated solution of Sodium Carbonate (15%). The overall mix was made up of 5ml using 2ml distilled water and then incubated for 20mins at 40°C. The absorbance of the resulting solution was read at 760nm in a UV visible spectrophotometer (Spectrum lab 752s). The total phenolic value was expressed in terms of Gallic acid equivalent (mg of Gallic acid/g of the extracted compound) (Chel-Guerrero et al., 2016).
Total flavonoid content:
Total flavonoid was determined using the method of Ordonez et al. (2006) for the formation of a complex flavonoid. A volume of 0.5 mL of 2% AlCl3-ethanol solution was mixed with 0.5 ml of the extract (1 mg/mL). The absorbance was measured at 420 nm using a UV-VIS spectrophotometer. Total flavonoid content was calculated as catechin equivalent (mg/100g) using the equation obtained from the curve Y=0.255x, R2=0.981 2, where x is the absorbance and Y is the catechin equivalent
DPPH scavenging assay
The method of Shen et al. (2010) was used for the determination of the scavenging activity of DPPH radicals in the extract solution. A portion (0.1ml) of 0.135 mM DPPH was prepared in methanol containing 0.5 mL of the extracts. The reaction mixture was vortexed thoroughly and thereafter left in the dark at room temperature for 30 min. The absorbance was measured spectrophotometrically at 517 nm. The scavenging ability of the food sample on DPPH was calculated using the equation:
DPPH scavenging activity (%) = [(Abs control – Abs sample)]/ (Abs control)] ×100,
Where Abs control is the absorbance of DPPH + methanol;
Abs sample is the absorbance of DPPH radical + sample extract or standard.
The results were expressed in % inhibition = DPPH scavenging activity
Total antioxidant capacity:
The total antioxidant capacity of the samples was analyzed using the method described by Prieto et al., (1999) with minor changes. An aliquot (0.3mL) of the sample extract was mixed with 3mL of the reagent (containing 0.6M sulphuric acid, 28mM sodium phosphate, and 4mM ammonium molybdate) and was incubated at 95°C for 90 mins. The mixture was cooled to room temperature and the absorbance was taken at 695nm spectrophotometrically using Optima SP-3000 (UV/VIS-SP-3000). The antioxidant capacity was expressed as ascorbic acid equivalent (AAE).
Ferric Reducing Antioxidant Potential (FRAP):
The reducing antioxidant potential of the sample was determined by assessing the ability of the sample to reduce iron chloride (Pulido et al., 2000). One milliliter of the sample (0.5g of the sample in 20ml ethanol) was added to 2.5ml of 200mM sodium phosphate buffer and 2.5ml of 1g/100ml potassium ferrocyanide. The absorbance of the mixture thereof was taken at 700nm indicating the reducing power of the sample. Decreased absorbance of the reaction mixture indicated higher reducing power of the sample extract.
Animal Study
Experimental design:
A total number of 35 Wistar albino rats were obtained from the Animal House, Physiology department of the University of Ibadan weighing between (95-110g). They were acclimatized for 10 days under controlled conditions: temperature of 30 ± 50 C, 55- 60% relative humidity with 12 hours light/ dark cycles. The rats were randomly selected into 7 groups, housed in standard cages, fed with standard rat feed (Ladokun feed) and water ad libitum after which the test diet was administered. The experiments were carried out following ethical clearance and directions obtained for this study from the Animal Care Unit and Research Ethical Committee, University of Ibadan, (ACUREC-UI).
Animal grouping and 3G snack food administration
The animals were selected into groups as follows.
Group (1): Negative Control: Rats unstressed and fed on standard Rat diet
Group (2): Positive Control: Rats stressed and fed on standard Rat diet
Group (3): Treatment 1: Rats stressed and fed on 10% Avocado seed-based 3G snack food
Group (4): Treatment 2: Rats stressed and fed on 20% Avocado seed-based 3G snack food
Group (5): Treatment 3: Rats stressed and fed on 30% Avocado seed-based 3G snack food
Group (6): Treatment 4: Rats stressed and fed on 100% Maize based 3G snack food
Group (7): Treatment 5: Rats stressed and fed on 100 % Avocado based 3G snack food
Induction of oxidative stress:
The method described by Al-Rejaie et al. (2012) was used with slight changes. Immobilization was used to induceoxidative stress in the animals by restraining each rat in a well-ventilated / plastic cage of the same size (2.5 in. X 4 in.) for 2 hours per day for 5 days in a week between the periods of 9 am and 12 noon. The movements of the control rats were however not restrained throughout the experimental period. When stress was being induced, the animals were deprived of food and water (Liu et al., 1996). The experiment lasted for 4 weeks.
Sample collection:
Four milliliters of blood samples were collected from each experimental rat by ocular puncture using heparinized capillary tubes. The blood was then dispensed into lithium heparin-coated tubes and centrifuged at 2000rpm for 15 mins. The plasma was separated and stored at -180 C and used for all the assays.
Biochemical assays
Estimation of malondialdehyde (MDA):
The method described by Al-Rejaie et al. (2012) was used for the estimation of malondialdehyde. One milliliter of 10 % trichloroacetic acid was mixed with two milliliters of plasma and boiled on a water bath for 20 mins. The resulting mixture was cooled and centrifuged at 3000 rpm for 15 mins. The optical density of the supernatant was next measured at 532nm using a spectrophotometer (Optima- 3000). The malondialdehyde (MDA) level was calculated using the molar extinction coefficient of (1.56 X 105 M-1 cm-1) (Arueya & Ugwu, 2017).
Measurement of antioxidant marker enzymes activity:
Determination of superoxide dismutase activity:
The method described by Oyedemi et al. (2010) was used to carry out this essay. The reaction mixture contained 0.5 ml of hepatic PMS (phenazinemethosulphate), 1 ml of 50 mM sodium carbonate, 0.2 ml of freshly prepared 0.1mM hydroxylamine hydrochloride, and 0.4 ml of 25 μm nitro blue tetrazolium. The reaction mixture was quickly mixed by inversion, following which a clear supernatant of 0.1ml of plasma (10% w/v) was added and centrifuged at 4000 rpm for 10 min. The change in absorbance was recorded at 560 nm.
Determination of catalase activity:
The method described by Onyeka et al. (2012) was used to estimate the catalase activity by measuring with a UV spectrophotometer the decrease in absorbance following decomposition of H2O2 at 240nm. The reaction mixture (3 ml) contained 2.9 ml of 30 mM H2O2 in phosphate buffer (pH 7.0) and 0.1 ml of serum in phosphate buffer (50 mM, pH 7.0). To calculate a reduction in absorbance, 240nm of 40M-1 cm-1 or extinction coefficient for H O was used. A mole of H2O2 reduced per minute per mg. protein was applied to express catalase activity.
Determination of glutathione:
The method was premised on the reduction of Ellman's Reagent (5,5’-dithio-bis- [2-nitrobenzoic acid]) (DTNB) with reduced glutathione (GSH) to generate a yellow compound. The reduced chromogen is directly proportional to GSH concentration and its absorbance measured at 405 nm using a commercial kit (Biodiagnostic, Egypt) (Noemann et al., 2011).
Liver function tests:
The standard procedure described by Burtis et al. (2007) was employed to determine the activities of Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), and Alanine phosphates (ALP). This was complemented using commercial Randox diagnostic kits (Randox Laboratories, USA).
Elucidation of the structure of possible bioactive compounds in the Snack
Following a slightly modified method of Ciudad-Mulero et al (2018), five grams of the sample were extracted in 30ml of hexane for 2 hrs, and the extracts filtered through cheesecloth. The filtrate was collected and re-filtered through Whatman paper No. 1. The resulting filtrate was evaporated under reduced pressure using a rotary evaporator at a low temperature of 40 ºC until a dry mass was obtained. The mass was collected and stored at 4 ºC for the elucidation of possible bioactive compounds. Before the elucidation, the mass extract was re-extracted with methanol before injection into gas chromatography-mass spectrometer (GCMS) (Model GC 7890A, MSD 5975). The column (Agilent 190915- 433) specifications were length 30 m, internal diameter 0.320 mm (i.d.), and thickness of 0.25 mm.
The elucidation of the identity of possible bioactive compounds was carried out using the method of Kim et al. (2007) with slight modification. An aliquot of 1.0 ml of the extract solution was injected. The identification of the components was done by comparing their Kovats GC retention indices and mass spectra with those obtained from the corresponding standards and data from the NIST MS library 14.
Sensory Evaluation
The sensory properties of the 3G snack food were evaluated using thirty (30) panelists (Iwe, 2002; Sharif et al., 2017). Each panelist was presented with different coded samples (max. four per session) of maize – avocado seed based 3G snacks. They were provided with a glass of water to rinse mouth in between sample evaluation. Sensory evaluation was carried out on a 9-point hedonic scale for Appearance, texture, taste, aroma, and overall acceptability (where 9=extremely liked and 1= extremely disliked).
Statistical analysis
One–way analysis of variance (ANOVA) SPSS version 20.0 software (Coakes, 2017) was used to analyze the data obtained in this study. The means were separated using Duncan's multiple range tests (p < 0.05) and presented as Mean ± standard deviation.
The seed of the avocado fruit can be utilized beneficially in the production of a functional food while also reducing its negative effect on the environment. This study revealed that the inclusion of avocado seed (up to 20%) in 3G snacks (see supplementary files) was most acceptable, safe, and exhibited a considerable impact on its weight loss and antioxidative potentials.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.