

Research Article - International Research Journal of Plant Science ( 2025) Volume 16, Issue 2
Published: 10-Apr-2025, DOI: http:/dx.doi.org/10.14303/irjps.2025.11
The goal of developing high-performing cultivars for organic production, driven by the declining performance of conventional germplasm in organic agriculture, is a crucial factor in closing the yield gap between conventional and organic farming. The on-going research at Cal Poly Pomona's Certified Organic Spadra Farm in Southern California, evaluated 72 F2 breeding lines of fresh market slicers, grape, cherry, and plum tomatoes in 2019. Ten superior lines with higher yield, and fruit quality were selected to advance into a replicated trail in 2020. We predicted a positive response to selection for the desired traits in this environment. Data were collected on fruit quality traits including scaring, and shape, growth habit, and yield, for both years, and dissolved soluble solids (BRIX) were collected in 2020. Analysis included ANOVA, multiple comparison testing, broad-sense heritability, correlations between traits, and response to selection. The significant (p<0.05) differences between the 2019 and 2020 seasons were found for all traits. However, response to selection showed that yield had responded negatively to selection indicating a possible influence of environmental factors while broad-sense heritability for yield was estimated at 0.22, suggesting remaining potential to select on yield. The fruit quality traits showed positive response to selection and highest broad-sense heritability estimated at 0.91 for stem scaring. Broad-sense heritability for BRIX was estimated at 0.67 and correlated to yield at R = 0.22 p<0.0001 for 2020 tomato breeding lines. Six of the best performing lines in 2020 were selected for advancement in the organic breeding program at Cal Poly Pomona for cultivar development.?
Organic production, plant breeding, heritability, fruit quality, yield, tomato, specialty crop, quantitative genetics.
Organic farmers have spent over 40 years refining and improving their agronomic systems, though organic farmers still relying on crop varieties developed in conventional highinput systems are estimated to be as high as 95% (Bueren, et al., 2011). Crop varieties tailored for organic and low input systems are few (Raggi, et al., 2017). When soybean cultivars bred for conventional systems were grown under organic management the cultivar was responsible for only 1% of the yield variability (Carkner & Entz, 2017). Trials on wheat found that populations selected in the organic environment attained higher yields at organic sites compared to populations selected at conventional sites (Kirk, et al., 2012; Murphy, et al., 2007). Desire for traits like yield, disease, pest, and stress resistance overlap between conventional and organic systems (Bueren, et al., 2011). The performance of conventional cultivars may be relying on fertilizers, fungicides, and genotype by environment interactions not available to the organic grower (Bueren, et al, 2011). Selection on traits benefited by resources unavailable to the organic tomato grower likely constrains the performance of the traits in the organic environment.
The tomato is known to have limited genetic variability that breeders can utilize, and this is evident in China where genetic distance can be as small as 0.0085 between cherry and slicer tomatoes (Chen, et al., 2009), partly due to the origin of the crop. Tomato (Solanum lycopersicum) originated in South America on the highlands along the western coasts of Peru and Chile. Its domestication is believed to have occurred in pre-Columbian times after migrating from the western coasts of South America to Mesoamerica (Smith, 2003). After early domestication by the Mesoamerican people, it was adopted by Europeans in the 15th century. Over the next hundred years, tomato was propagated around the world, from Mesoamerica, to the Caribbean, mainland Europe and eventually as far as the Philippines and mainland Asia (Smith, 2003). Its migration across the world led to a series of selections that resulted in several genetic geographical isotopes. Additionally, the development of self-compatible tomatoes led to tomatoes which could fertilize themselves resulting in varieties able to inbreed (Bai & Lindhout, 2007). The result of this inbreeding resulted in steady reduction in genetic diversity even without selection pressures being applied. The popularity of the tomato has enabled its traversal across the world, but at the cost of its genetic variation. The genetic distinctness between types of tomatoes, including market fresh, cherry, and grape is still sufficient to distinguish them by DNA sequencing (Phan et al., 2016). Further increasing the genetic diversity of tomatoes is attributed to the effort of breeders' introgression of wild species (Phan et al., 2016). A necessary process as outcrossing by domestic tomatoes is generally low and cultivar dependent, reinforcing the roll of introgression of wild genes into cultivated tomato germplasm (Horneburg & Becker, 2018). The ability of the tomato to outcross may be desirable and feasible to develop for the organic farmer as options for seed production are desired by the Organic Seed Alliance (Horneburg & Becker, 2018; Bueren, et al., 2011).
Research on tomato fruit quality has examined shelf life, sugar, acid, flesh color, anthocyanin, carotenoids, pericarp proteins, aromatics, and which of these traits is most amenable to alteration through breeding (Breksa, et al., 2015; Lee, et al., 2019; Rodriguez, et al., 2011; Sestari, et al., 2014; Yuan, et al., 2008; Jones & Scott, 1983; Garg, et al., 2008; Graham, et al., 1999). Fruit quality traits are primarily quantitative traits showing a wide range of diversity (Bertin & Genard, 2018). Tomato flavor was found to be strongly influenced by the sugar and acid content of the fruit which are linked to soluble solids (Jones & Scott, 1983). Breeding for increased soluble solids is recommended for improving the flavor of the tomato (Jones & Scott, 1983). Fruit scaring in tomato reduces the fruits quality and marketability Blossom-end scaring was a trait that could readily be controlled through selection (Elkind, et al., 1990).
Yield in tomato varieties is a complex trait and governed by the high genotype x environment interaction, while fruit shape traits are less affected (Figas, et al., 2018). In organic field cultivation it was noted that fruit cracking occurred more frequently and possibly contributed to reduced yield as soil moisture may have varied more than in a conventional system (Figas, et al., 2018). Additive gene effects were found to contribute the greatest to yield in tomatoes, while non-additive gene effects play a role in fruit number and yield but to a lesser effect than additive genes (El-Gabry, et al., 2014; Martinez-Vazquez, et al., 2017). Selection for fruit mass and other yield related traits may complicate the selection process as adjacent genes may control metabolites influencing the appearance and taste of the tomato (Zhu, et al., 2018).
In California, the tomato industry is responsible for production of 95% of the nation's processed tomatoes, a third of the nation's market fresh tomatoes, and a third of the world's total tomato production (National Agricultural Statistics Service, 2018). The popularity of organic produce in California can been seen as well as it was responsible for 66% of the nation's organic vegetable production and 61% of all organic tomatoes in 2008. This popularity has only grown continuing to increase with organic crops reaching a value of 2.1 billion USD in California in 2016. The value of organic tomatoes grown in the open in California was reported as 122 million USD in 2016 by the USDA (National Agricultural Statistics Service, 2017). Improving the yield and fruit quality of organic tomatoes is highly desirable for supporting this emerging market and thousands of farms in the United States (National Agricultural Statistics Service, 2019).
Organic agriculture is considered the most sustainable low input model as an alternative to the inherent adverse association with chemicals in the agricultural system. Such agricultural method adheres to the principles of; integrated pest management; rotation of cropping systems to minimize weeds, disease and insect populations; minimizing soil erosion, reducing water loss; the use of organic fertilizers and green manures (Healy et al., 2017; Campanelli et al., 2015; Shaver, 2003). The principles of organic agriculture are centered on maintaining sustainable biodiversity within local ecosystems, and greater integration of crop and livestock production systems (Lammertts van Bueren, 2011; Mader et al., 2002).
Demand for organic products is partially driven by the acceptance that organically grown products are healthier and more nutritious than conventionally grown products (Lammertts van Bueren, 2011; Lotter, 2003). Organic farming systems have focused on optimizing agronomic approaches for the last 40 years. There is now a greater focus shifting to the optimizing of genetic improvements to enhanced yield stability under low-input conditions (Lammertts van Bueren, 2011). A solution to low yields in organic agriculture is organizing breeding programs to directly select varieties grown in low input organic systems specifically adapted to the local microclimate (Campanelli et al., 2015; Murphy et al., 2007; Wolfe et al., 2008;). Expression of selected traits under organic systems in the population generations will determine the heritability of the traits in tomatoes.
The seed used originated from crosses made by Seed Revolution Now, an Organic Seed Alliance collaborator working with Cal Poly Pomona. The F1 generations were grown, and bulk harvested in 2018 at a certified organic farm site within Cal Poly Pomona (CPP). Trials at CPP in 2019 and 2020 consisted of 72 F2 lines that were grown out and evaluated in head rows. With 10 of the best performing lines being selected for a RCBD trial in 2022. The field management of the trials was a low input organic system in compliance with the National Organic Program list of Allowed and Prohibited substances. At Cal Poly Pomona, tomatoes breeding lines were seeded in February, transplanted to the field in late April, and Harvested in July and August.
Greenhouse Transplant Seeding
Seventy-two-cell seedling trays were prepared by filling them with Kellogg organic gardening soil (Kellogg Garden Products Corporate), a National Organic Program compliant organic input material. The material was passed through a 6mm sieve to remove any coarse material present in the potting medium. The trays cells were seeded each with a single seed and topped with tray medium and then vermiculite. In the second year, the number of experimental populations was doubled at the time of seeding. The trays were monitored daily, in a temperature regulated greenhouse between 20 to 30-degrees Celsius located at CPP. Germination data was collected weekly until transplanting. 5-1-1 Alaska fish emulsion (Central Garden & Pet) was applied at label rate seven weeks after germination. When 90% of the seedlings were sufficiently developed (emergence of 4th leaf), they were transplanted at CPP's Spadra farm's certified organic research field.
Experiment and Field Design
In 2019, the field planting consisted of twenty-four raised beds 22 cm tall and 1.2 meter wide. With 0.6 meters of space between each bed with the center lines of the beds being 1.8 meter apart. Soil samples were collected and tested for nutrient deficiencies and soil pH. 5-1-1 Alaska fish emulsion (Central Garden & Pet) and Dr. Earth Golden Bloom (Dr. Earth, CO), MicroPak (Advancing Eco Agriculture, LLC) and Rebound Iron (Advancing Eco Agriculture, LLC) were applied at label rates at the start of transplanting after the soil testing showed NPK and micronutrient deficiency. Drip tape was run down the center of the bed and irrigation was scheduled to maintain soil moisture in an eight-inch root zone. Trellising stakes and twine were then placed down the length of beds. Head row spacing between tomatoes was 45 cm apart, with each plant tracked individually and identifier created based on its position within the field.
In 2020, ten lines selected from individual plants in 2019 were grown in a randomized complete block design (RCBD) with two trials, each containing three blocks. Each block had twelve plots, and each plot included twelve plants. Due to shortage of labor during COVID-19 pandemic, plants were restricted to only three vines for the first month in the field.
Harvesting and fruit data collection started in July 2019 and 2020. Just before or during harvest, fruit quality data were gathered, including measurements of fruit firmness, brix, stem attachment, stem scar, blossom end scarring, fruit cracking, fruit ripening, and fruit morphology traits. In addition, yield, number of fruits per plant, plant height, and fruit weight per plant were also recorded during harvest. The data was recorded on preformatted data sheets and transferred to Microsoft Excel.
Data Collection, Analysis, and Selection
Data were collected and recorded in Microsoft Excel for each individual plant in both years for every trait listed in table 1, which were carded for analysis in SAS OnDemand for Academics (SAS Institute Inc., 2021), and R Studio 2022.07.1 b554 (RStudio PBC., 2022). For 2019 each line's population was randomly sampled for comparison and selection, the necessary sample size was calculated with G*Power 3.1.9.2 (Heinrich-Heine-Universitat Dusseldorf, 2021). In 2020 data was collected for all plants in the experiment. Pearson correlation coefficients were also calculated for each possible pair of traits. Welch's ANOVA was conducted and post-hoc Tukey was used for multiple comparisons of lines. Kruskal- Wallis was used to test differences between means and post-hoc Dwass, Steel, Critchlow-Flinger method was used for multiple comparison analysis of 2020 fruit quality data, and for comparisons between lines for 2019 and 2020. For all tests significance was established at p < 0.05. Broadsense heritability was calculated in R (version 4.3.0 2023-04-21). Response to selection was calculated in Microsoft Office Excel 360 from the formula. Results of the analysis were used to inform the ongoing selection of material at the Organic Tomato Breeding Program at Cal Poly Pomona, CA. (Figure 1) and Table 1).
| Generations Evaluated | Parent Line | Progeny Line | Market Type |
|---|---|---|---|
| F2 & F3 | Jaepak Red | 10-114 | Round Slicer |
| F2 & F3 | Royal Iris | 12-003 | Plum |
| F2 & F3 | Bronco Red | 12-069 | Plum |
| 12-073 | Plum | ||
| F2 & F3 | Satsuma Cherry | 12-115 | Cherry |
| F2 & F3 | Kermes Round | 01-011 | Round |
| 01-047 | Round | ||
| F2 & F3 | PS18-JAKE | 01-079 | Slicer |
| 01-083 | Slicer | ||
| 01-096 | Slicer |
For ten lines grown in 2020 significant differences were found between lines for fruit stem scar, fruit blossom end scaring, fruit cracking, uniformity of ripening, yield, brix, and fruit shoulder smoothness. Broad-sense heritability and response to selection was calculated for all traits except BRIX, for which only H was calculated. The correlation between yield and BRIX, stem and blossom end scarring, fruit cracking and fruit shoulder smoothness, as well as brix and uniformity of ripening.
For all traits except yield and BRIX Kruskal-Wallis tests were conducted with a significant p-value threshold of 0.05. If significant results were found it was followed by a Dwass, Steel, Critchlow- Flinger method for multiple comparisons of the lines. Yield and Brix significant differences were tested with Welch's ANOVA and multiple comparisons made with Tukey's HSD Test. Data on stem attachment or the jointing of the pedicle was gathered but not analyzed due to its infrequency in the population.
Between ten lines grown in 2020 significant difference (p < 0.0001) was found for stem scaring. Multiple comparisons revealed Royal Iris 12-003, Satsuma Cherry 12-115 and Bronco Red 12-073 as having the least amount of stem scaring. Comparisons between the 2019 parents and 2020 progeny found significant differences for Bronco Red, Jaepak Red, Satsuma Cherry and their progeny. Significant difference (p < 0.0001) was found between ten lines grown in 2020 for blossom end scaring, revealing Royal Iris 12-003 and Satsuma Cherry 12-115 as having the least amount of stem scaring. Comparisons between the 2019 parents and 2020 progeny found significant differences for Bronco Red, Jaepak Red, Satsuma Cherry, Kermes Round, and PS18-JAKE with the progeny improving.
We found significant differences (p<0.0001) between ten lines in 2020 for fruit cracking. Comparisons revealed Royal Iris 12-003, Jaepak 10-114, Satsuma Cherry 12-114, and Bronco Red 12-073 as having the least amount of stem scaring. Comparisons between the 2019 parent and 2020 progeny lines found significant differences for Royal Iris, Bronco Red, Satsuma Cherry and their progeny. The difference between Kermes Round and PS18-JAKE and their progeny was in a negative direction between generations. Significant difference (p < 0.0001) was found between ten lines grown in 2020 uniformity of ripening. Comparisons revealed Bronco Red 12-069, PS18-JAKE 01-083, and PS18-JAKE 01-078 as having the most uniform ripening in the 2019 population. Comparisons between 2019 and 2020 lines found significant differences for Jaepak Red, Satsuma Cherry, Kermes Round, PS18-JAKE and their progeny. However, the difference was in a negative direction for all the progeny with no improvement of the trait between generations. Significant difference (p < 0.0001) was found between ten lines grown in 2020 for shoulder smoothness trait. Comparisons revealed Royal Iris 12-003, Satsuma Cherry 12-115, Bronco Red 12-073 as having the smoothest fruit in the 2019 population. Comparisons between the 2019 and 2020 lines found significant differences for Royal Iris, Bronco Red, PS18-JAKE and their progeny. The direction was negative for all progeny except PS18-JAKE which showed an improvement in the trait between generations.
A significant difference (p < 0.0001) was found between the 10 lines grown in 2020 for yield. Multiple comparisons revealed 3 means groupings with Kermes Round 01-047 having the highest yield measurements. Comparisons between the 2019 and 2020 lines found significant differences for all parent lines and progeny.
A significant difference (p < 0.0001) was found between ten lines grown in 2020 for yield. Multiple comparisons revealed three means groupings with Kermes Round 01-047 having the highest yield measurements. Comparisons between 2019 and 2020 lines found significant differences for all parent lines and progeny.
However, the differences between parents and offspring were in a negative direction showing no improvement of the trait between generations. Significant difference (p < 0.0001) was found between ten lines grown in 2020 for Brix. Multiple comparisons revealed five means groups with Satsuma Cherry 12-115 having the highest Brix measurements. No comparisons between 2019 and 2020 lines were made as Brix data was not collected for the 2019 parent lines.
Heritability and Response to Selection
Broad sense heritability was calculated as 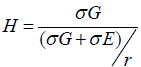 using a linear model built in R version 4.3.0 using the
lme4 package version 1.1-33. Response to selection was
calculated as
using a linear model built in R version 4.3.0 using the
lme4 package version 1.1-33. Response to selection was
calculated as
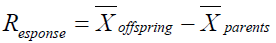
Relatively high broad sense heritability was found for BRIX, stem scaring, blossom end scaring, fruit cracking and shoulder smoothness. Yield and uniformity of ripening had the lowest heritability of all the traits analyzed. Yield, blossom end scaring and ripening had an unexpected negative response to selection. While stem-scraping, fruit cracking, and smoothness all showed a positive response to selection (Table 2) and (Figure 2).
| Statistic | Yield | BRIX | Stem Scar | Blossom End Scar | Cracking | Ripening | Smoothness |
|---|---|---|---|---|---|---|---|
| Heritability | 0.22 | 0.67 | 0.89 | 0.92 | 0.79 | 0.24 | 0.93 |
| Response to Selection | -0.16 | 0.48 | 0.91 | 0.08 | 0.60 | 0.88 |
Four correlations were examined between yield and BRIX, stem and blossom end scaring, fruit smoothness and cracking, and BRIX and ripening. All four correlations across the population were found to be significant at p<0.05. Yield and BRIX correlated at R=0.22, p<0.0001, surprising us as literature often reports these traits as having no or negative correlations. These correlations across the population and individual lines are documented in. (Figure 2, 3 and 4).
The current project examined six F2 lines selected from a base population of seventy-two lines, and ten F3 descendant progeny lines (Table 1) over a period of two years (plantings). The lines were evaluated for qualitative fruit characteristics including stem and blossom end scaring, fruit cracking, uniformity of ripening, fruit shoulder smoothness, quantitative traits: yield and BRIX. Significant differences were found in performances between the organic tomato breeding lines in 2019 F2 to F3 2020 generations. Environmental conditions were naturally not uniform between seasons and varied in each year. In addition, the incidence of COVID-19 pandemic impacted the effective utilization of resources and timely field work. New protocols were implemented to ensure safety compliance and project feasibility in 2020.
Significant Differences
Comparisons in fruit quality between the 2019 parent generation and 2020 progeny showed fewer differences between generations in fruit shoulder smoothness. With only three out of the ten progeny lines having smoother fruit than their parents and six out of ten staying close to their parents high scoring means. While scaring was reduced in eight of the ten progeny lines compared to the parents, and four out of ten progeny lines had reduced fruit cracking compared to the parent populations. With two of the six remaining progeny lines observed near the parents high scoring mean. The distribution of scores for these traits showed a reduction in variability between generations.
High performing lines for yield were not consistent between years suggesting the possibility of different tolerances to heat stress between the lines. Specifically, Jaepak Red 10-114 falls into a different means grouping than Kermes Round 01- 047 and PS18-JAKE 01-096 (lines whose parents were high performers for yield in 2019). These results correspond with other findings of high genotype x environment interaction for yield (Figas, et al., 2018). The difficulty of developing abiotic tolerances with the limited genetic variability of the tomato (Zsogon, et al., 2017; Chen, et al, 2009). Finally, among ten progeny lines, five means groups appeared for BRIX, finding three groups to be significant without overlap.
The response to selection and heritability was favorable for fruit quality traits. Generally, blossom-end scaring, stem scaring, shoulder smoothness and fruit cracking were readily improved or maintained through a selection intensity of 10%. It is likely that limits in the scoring scale reduced the ability to differentiate high performing parents and progeny by their means scores. Of ten progeny lines nine showed a positive response to selection with reduced blossom end scaring compared to the parent populations. The ability to reduce blossom-end scaring through selection (Elkind et al., 1990) appears to be extendable to stem scaring as well from our results. The ease of inheriting sufficiently low fruit stem scar suggests minimal effort is needed to maintain high performance over generations in this trait. The correlation between blossom-end scaring and stem scaring, as well as between shoulder smoothness and fruit cracking provides may provide an opportunity for simplifying selection by focusing on the highest priority trait in these pairings.
Yield, Uniformity of Ripening, and BRIX
Uniformity of ripening and, more importantly, yield did not show expected results from early generation selection. The uniformity of ripening showed a negative response to selection, this may be due to a variety of compounding factors, including weather, or genetic diversity. Similarly, fruit yields did not improve between generations also showing a negative response to selection, initially attributing this to strong weather effects (heat stress) during the summer of 2020. In addition to changes in cultivation practices to accommodate the reduced labor availability during the 2020 COVID-19 outbreak. However, this result agrees with papers discussing the efficacy of selection in early generations with the presence of large nongenetic effects (Bernardo, 2003; Yang 2009). Development of well combining inbreed lines adapted to organic tomato production may be a more reliable method for deriving yield gains for organic production compared to early generation selection. The positive correlation between yield and BRIX in 2020 was found and may be the result of the underperformance of yield in the 2020 season. While BRIX testing was only conducted in 2020, it is planned to continue as part of the evaluation of future generations. However, the distribution of the trait's performance was wide within the populations, suggesting sufficient variance was present in the populations and supported by improvement of the BRIX trait in later generations.
Breeding objectives for fruit quality were either met or showed improvement in the majority of the selected breeding lines in the current research project. While breeding goals for yield and uniformity of ripening may be better met with selection in later generations. The results of Brix testing highlighted a future opportunity to not just meet and improve on the breeding objectives of this research project but a chance to produce a market relevant variety for organic tomato growers. Several outstanding breeding lines were identified. In terms of fruit quality Royal Iris, Bronco Red, and Satsuma Cherry have exceptionally smooth fruit, free of blemishes, cracking and scaring. Brix scoring indicative of sugar and acid content is especially promising for the lines Satsuma Cherry, Kermes Round 01- 047, and PS18-Jake 01-096. Potential remains in the high yielding lines Kermes Round 01-047 and PS18-Jake 01-096. After an extreme heatwave individual plants from these lines still yielded over 1kg of fruit. Work with these lines continues towards the development of potential varietal releases.
Organic tomato breeders will continue to develop strategies that are likely to remain closer to the modern combination of traditional and genomic techniques. The ease of which some fruit quality traits can be improved through early selection, as well as the difficulty of improving yield a trait influenced by a large amount of additive gene effects and high genotype x environmental interaction, (Martinez, Vazquez, et al., 2017; Figas, et al., 2018). Creating hybrid lines of organic tomatoes by selecting parents with good combining ability for fruit quality with parents with high yield characteristics may be an approachable strategy to produce a superior line with higher yield and fruit quality for the smaller breeding operation. Improving the fruit quality of an already high yielding inbreed line may offer additional benefit to the breeder in reducing the complexity of field trials for smaller breeding programs. Selection would focus on less laborintensive evaluation of fruit quality, especially operations which employ hand harvesting. Additionally, development of new and more accessible remote and proximal sensing tools is desirable for smaller seed companies, labs, and communities. As the effort to manage and measure large breeding populations may be limiting the participation of the wider organic farming community. In orientating towards the future, we plan continued investigation into the observed positive correlation between yield and brix and if it will remain in later generations. Additionally, we are interested in determining the presence of genetic linkages in tomato breeding populations to enhance fruit quality traits and develop superior performing lines for commercial purposes.
Bai, Y., & Lindhout, P. (2007). Domestication and breeding of tomatoes: what have we gained and what can we gain in the future?. Annals of botany, 100(5), 1085-1094.
Indexed at, Google Scholar, Cross Ref
Bertin, N., & Génard, M. (2018). Tomato quality as influenced by preharvest factors. Scientia Horticulturae, 233, 264-276..
Indexed at, Google Scholar, Cross Ref
Breksa III, A. P., Robertson, L. D., Labate, J. A., King, B. A., & King, D. E. (2015). Physicochemical and morphological analysis of ten tomato varieties identifies quality traits more readily manipulated through breeding and traditional selection methods. Journal of Food Composition and Analysis, 42, 16-25.
Indexed at, Google Scholar, Cross Ref
Bueren, E. L., Jones, S. S., Tamm, L., Murphy, K. M., Myers, J. R., Leifert, C., & Messmer, M. M. (2011). The need to breed crop varieties suitable for organic farming, using wheat, tomato and broccoli as examples: A review. NJAS-Wageningen Journal of Life Sciences, 58(3-4), 193-205.
Indexed at, Google Scholar, Cross Ref
Campanelli, G., Acciarri, N., Campion, B., Delvecchio, S., Leteo, F., Fusari, F., ... & Ceccarelli, S. (2015). Participatory tomato breeding for organic conditions in Italy. Euphytica, 204(1), 179-197.
Indexed at, Google Scholar, Cross Ref
Carkner, M. K., & Entz, M. H. (2017). Growing environment contributes more to soybean yield than cultivar under organic management. Field Crops Research, 207, 42-51.
Indexed at, Google Scholar, Cross Ref
Chen, J., Wang, H., Shen, H., Chai, M., Li, J., Qi, M., & Yang, W. (2009). Genetic variation in tomato populations from four breeding programs revealed by single nucleotide polymorphism and simple sequence repeat markers. Scientia Horticulturae, 122(1), 6-16.
Indexed at, Google Scholar, Cross Ref
El-Gabry, M. A. H., Solieman, T. I. H., & Abido, A. I. A. (2014). Combining ability and heritability of some tomato (Solanum lycopersicum L.) cultivars. Scientia Horticulturae, 167, 153-157.
Indexed at, Google Scholar, Cross Ref
Elkind, Y., Galper, O. B. O., Scott, J. W., & Kedar, N. (1990). Genotype by environment interaction of tomato blossom-end scar size. Euphytica, 50, 91-95.
Indexed at, Google Scholar, Cross Ref
Figàs, M. R., Prohens, J., Casanova, C., Fernández-de-Córdova, P., & Soler, S. (2018). Variation of morphological descriptors for the evaluation of tomato germplasm and their stability across different growing conditions. Scientia Horticulturae, 238, 107-115.
Indexed at, Google Scholar, Cross Ref
Garg, N., Cheema, D. S., & Dhatt, A. S. (2008). Genetics of yield, quality and shelf life characteristics in tomato under normal and late planting conditions. Euphytica, 159, 275-288.
Indexed at, Google Scholar, Cross Ref
Graham, R., Senadhira, D., Beebe, S., Iglesias, C., & Monasterio, I. (1999). Breeding for micronutrient density in edible portions of staple food crops: conventional approaches. Field crops research, 60(1-2), 57-80.
Indexed at, Google Scholar, Cross Ref
Healy, G. K., Emerson, B. J., & Dawson, J. C. (2017). Tomato variety trials for productivity and quality in organic hoop house versus open field management. Renewable Agriculture and Food Systems, 32(6), 562-572.
Indexed at, Google Scholar, Cross Ref
Horneburg, B., & Becker, H. C. (2018). Spontaneous outcrossing in tomato depends on cultivar and environment and varies between individual flowers. Plant Breeding, 137(4), 638–643.
Indexed at, Google Scholar, Cross Ref
Jones, R. A., & Scott, S. J. (1983). Improvement of tomato flavor by genetically increasing sugar and acid contents. Euphytica, 32, 845-855.
Indexed at, Google Scholar, Cross Ref
Kirk, A. P., Fox, S. L., & Entz, M. H. (2012). Comparison of organic and conventional selection environments for spring wheat. Plant Breeding, 131(6), 687-694.
Indexed at, Google Scholar, Cross Ref
Lammerts van Bueren, E. T., Backes, G., De Vriend, H., & Østergård, H. (2010). The role of molecular markers and marker assisted selection in breeding for organic agriculture. Euphytica, 175, 51-64.
Indexed at, Google Scholar, Cross Ref
Lee, J. H., Jayaprakasha, G. K., Avila, C. A., Crosby, K. M., & Patil, B. S. (2019). Metabolomic studies of volatiles from tomatoes grown in net-house and open-field conditions. Food Chemistry, 275, 282-291.
Indexed at, Google Scholar, Cross Ref
Lotter, D. W., Seidel, R., & Liebhardt, W. (2003). The performance of organic and conventional cropping systems in an extreme climate year. American Journal of Alternative Agriculture, 18(3), 146-154.
Indexed at, Google Scholar, Cross Ref
Mäder, P., Fliessbach, A., Dubois, D., Gunst, L., Fried, P., & Niggli, U. (2002). Soil fertility and biodiversity in organic farming. Science, 296(5573), 1694-1697.
Indexed at, Google Scholar, Cross Ref
Martínez-Vázquez, E. de los Á., Hernández-Bautista, A., Lobato-Ortiz, R., García-Zavala, J. J., & Reyes-López, D. (2017). Exploring the breeding potential of Mexican tomato landraces. Scientia Horticulturae, 220, 317–325.
Indexed at, Google Scholar, Cross Ref
Murphy, K. M., Campbell, K. G., Lyon, S. R., & Jones, S. S. (2007). Evidence of varietal adaptation to organic farming systems. Field Crops Research, 102(3), 172-177.
Indexed at, Google Scholar, Cross Ref
Phan, N. T., Kim, M. K., & Sim, S. C. (2016). Genetic variations of F1 tomato cultivars revealed by a core set of SSR and InDel markers. Scientia Horticulturae, 212, 155-161.
Indexed at, Google Scholar, Cross Ref
Raggi, L., Ciancaleoni, S., Torricelli, R., Terzi, V., Ceccarelli, S., & Negri, V. (2017). Evolutionary breeding for sustainable agriculture: Selection and multi-environmental evaluation of barley populations and lines. Field Crops Research, 204, 76-88.
Indexed at , Google Scholar, Cross Ref
Rodríguez, G. R., da Costa, J. H. P., Tomat, D. D., Pratta, G. R., Zorzoli, R., & Picardi, L. A. (2011). Pericarp total protein profiles as molecular markers of tomato fruit quality traits in two segregating populations. Scientia Horticulturae, 130(1), 60-66.
Indexed at, Google Scholar, Cross Ref
Sestari, I., Zsögön, A., Rehder, G. G., Teixeira, L. de L., Hassimotto, N. M. A., Purgatto, E., Benedito, V. A., & Peres, L. E. P. (2014). Near-isogenic lines enhancing ascorbic acid, anthocyanin and carotenoid content in tomato (Solanum lycopersicum L. cv Micro-Tom) as a tool to produce nutrient-rich fruits. Scientia Horticulturae, 175, 111–120.
Indexed at, Google Scholar, Cross Ref
Shaver, J. M. (2003). Toward a greener agriculture. Plant, genes and crop biotechnology, 473-499.
Smith, A. F. (2003). Tomato. In S. H. Katz, Encyclopedia of Food and Culture (pp. 402-407). New York, NY: Charles Scribner's Sons.
Wolfe, M. S., Baresel, J. P., Desclaux, D., Goldringer, I., Hoad, S., Kovacs, G., ... & Lammerts van Bueren, E. T. (2008). Developments in breeding cereals for organic agriculture. Euphytica, 163, 323-346.
Indexed at, Google Scholar, Cross Ref
Yang, R. C. (2009). When Is Early Generation Selection Effective in Self‐Pollinated Crops?. Crop Science, 49(6), 2065-2079.
Indexed at, Google Scholar, Cross Ref
Yuan, D., Chen, J., Shen, H., & Yang, W. (2008). Genetics of flesh color and nucleotide sequence analysis of phytoene synthase gene 1 in a yellow-fruited tomato accession PI114490. Scientia horticulturae, 118(1), 20-24.
Indexed at, Google Scholar, Cross Ref
Zhu, G., Wang, S., Huang, Z., Zhang, S., Liao, Q., Zhang, C., ... & Huang, S. (2018). Rewiring of the fruit metabolome in tomato breeding. Cell, 172(1), 249-261.
Indexed at, Google Scholar, Cross Ref
Zsögön, A., Cermak, T., Voytas, D., & Peres, L. E. P. (2017). Genome editing as a tool to achieve the crop ideotype and de novo domestication of wild relatives: case study in tomato. Plant Science, 256, 120-130.