

Research Article - International Research Journal of Agricultural Science and Soil Science ( 2021) Volume 10, Issue 5
Received: 06-Jul-2021 Published: 21-Sep-2021
An investigation was carried out during two consecutive years (2019 and 2020) at Seed Research Farm, Department of Seed Science and Technology, H.N.B. Garhwal University, Srinagar Garhwal, Uttarakhand (India). The experiment was conducted on the effect of plant growth-promoting rhizobacteria on seed quality parameters of Finger millet (Eleusine coracana L.). There were eight treatments including control and each treatment was replicated thrice. The data was analyzed in randomized block design. The study revealed that all the seed quality parameters like 1000 seed weight (2.46 g), Seed protein content (26.06 μg/mg), Seed Nitrogen content (4.22%), Phosphorus content (0.931%), Potassium content (0.503%), seed germination (95.50%), seedling length (9.58 cm), seedling dry weight (4.73 mg), seed vigour index-I (SVI-I) (890.94) and seed vigour index-II (SVI-II) (439.89) were recorded maximum with treatment of PGPR, alone and in the combination of two strains. All parameters showed minimum values in untreated control as compared to the rest of the treatments. The study revealed that all the treatments of PGPR had a significant impact on seed quality parameters of finger millet.
Seed quality parameters, PGPR.
Finger millet (Eleusine coracana L) is a crop of the arid and semi-arid region of eastern and southern Africa, India and Nepal belong to the family Poaceae, which is locally named as ragi or mandua. It is one of the rich sources of minerals such as calcium, iron, and manganese. The crop is widely recommended to diabetic patient and people with digestive disorders as it is gluten-free and contains low glycemic index and fibres. The finger millet crop has wider applicability to be used as a nutrition source in food and straw for livestock feed. Effective fertilizer recommendation is the need of time to ensure the quality produce and higher yield along with sustainable utilization of resources. To meet the growing demand of the burgeoning population, large amounts of herbicides, pesticides and fertilizers are being applied to the fields every year to achieve maximum production. The use of chemicals in Indian agriculture has increased 170 times in the last 50 years (FAO, 2010). This is now a major environmental and health concern because of the deleterious impact of these chemical compounds on terrestrial and aquatic ecosystems. To sustain the efficiency of soil, crop yield and reduce dependency on chemical fertilizers, the combined use of organic chemical and biofertilizers is very much essential (Kumar et al., 2003).
Biofertilizers are substances which contain living microorganisms, when it is applied to seed, plant surface and soil, colonize the rhizosphere or the interior of the plant and promote plant growth by increasing the supply or availability of primary nutrients to the host. These are an environmentally friendly, renewable and potential source for plant nutrients (Vessey, 2003; Bhattacharjee, Dey, 2014; Raghuwanshi, 2012). They are enhanced soil fertility, improve soil structure, and add organic matter to the soil. Biofertilizers significantly increased plant growth, yield and quality parameters of crops (Youssef and Eissa, 2014).
Different species of PGPRs such as Azospirillum, Azotobacter, Bacillus, Enterobacter and Pseudomonas, are used as biofertilizers for different crops (Rokhzadi and Toashih, 2011).
Pseudomonas species are an ecologically significant group of bacteria for plant growth on the earth. They comprise the genus, P. aureofaciens, P. chlororaphis, P. fluorescens, P. putida and the plant pathogenic species P. cichorii and P. syringae (Dwivedi and Johri, 2003). Fluorescent pseudomonas is reported well as phosphate solubilization, plant growth-promoting bacteria and as a biocontrol agent against different soil and seed born plant pathogens.
The use of Bacillus amyloliquefaciens species of bacteria in the soil as well as seed treatment can enhance the available form of soil nutrient in the rhizosphere, and enhanced plant growth and induced pest defense systems (Garcia et al., 2015). Available Soil nutrient significantly influenced seed germination and plant growth. PGPR enhanced nutrient bioavailability in the rhizosphere and absorb by the plant through root transporters (De-Willigen, 1986; Bidondo et al., 2012).
The use of Bacillus amyloliquefaciens species of bacteria in the soil as well as seed treatment can enhance the available form of soil nutrient in the rhizosphere, and enhanced plant growth and induced pest defense systems (Garcia et al., 2015). Available Soil nutrient significantly influenced seed germination and plant growth. PGPR enhanced nutrient bioavailability in the rhizosphere and absorb by the plant through root transporters (De-Willigen, 1986; Bidondo et al., 2012).
The present investigation was carried out in 2019 and 2020 at the Seed research farm of the Department of Seed Science and Technology, H.N.B. Garhwal University, Srinagar Garhwal, Uttarakhand (India). The seed of finger millet was collected from Silkakhal village of Tehri Garhwal district (Uttarakhand). Three strains of PGPR are used in this experiment; two are Bacillus amyloliquefaciens (BS-27, BS-27) and one is Pseudomonas (Y-19). The strains were procured from the Department of Microbiology, College of Forestry, UUHF University Ranichauri campus Tehri Garhwal, Uttarakhand. The previous studies confirmed the plant growth activity of the selected Bacillus and pseudomonas strains (Negi, 2011; Panday, 2018). Again the procured bacterial strains were checked for their plant growthpromoting activity on finger millet under field conditions.
The experiment was laid out in randomized block design (RBD) with seven treatments with control, replicated thrice. The treatments were T0: Untreated control, T1: BS-27, T2: BS-58, T3: Y-19, T4: BS-27+BS-58, T5: BS-58+Y-19, T6: BS- 27+Y-19, T7: BS-27+ BS-58+Y-19. PGPR strains applied 10g kg-1 of seeds as seed inoculation under field sowing.
Protein content in finger millet seed
The protein content of seed was estimated as a method suggested by Lowry et al., (1951).
Nitrogen content in seed
To determine nitrogen content in seed, ground samples from each plot were digested and analyzed separately adopting modified Kjeldahl’s method given by (Piper, 1966). Nitrogen was estimated by digesting 0.5 g seed with 10 ml concentrated sulphuric acid in the presence of catalyst mixture Selenium dioxide, Copper sulphate and Potassium sulphate.
Phosphorus and potassium content in seed
To determine phosphorus and potassium content 0.5 g sample was wet-digested in a triple-acid mixture of HNO3, HClO4 and H2SO4 in the ratio of 9:3:1 as outlined by (Piper, 1966). The phosphorus content was determined to process suggested (Jackson, 1967) using by Spectrophotometer at 470nm wavelength. Potassium content was determined by the Flame photometer method.
Seed germination test
Seed germination and seedling growth parameters such as germination percent, seedling length, seedling dry weight, 100 harvested seeds from all replications of each treatment were used for conducting the germination test as per ISTA rules. This was carried out by using the top of paper method in the seed germinator at 25°C. The final counts were taken after 8 days.
The observation for various germination and seedling growth attributes such as Germination percent, seedling length, seedling fresh weight, seedling dry weight, were recorded after eight days.
All parameters of germination and seedling growth was recorded After the above-mentioned observations were recorded other seed germination parameters were calculated as follows:
1. Seed Germination Percent (Gairola et al., 2011)
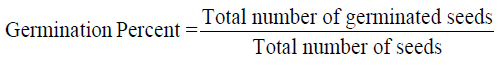
2. Speed of Germination (Maguire, 1962)
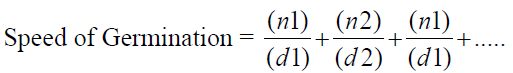
Where n= number of germinated seed and d = number of days
3. Mean Daily Germination (Scott et al.,1984)
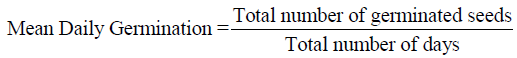
4. Seedling Vigor Index I (Abdul Baki and Anderson, 1973)
Seedling Vigor Index I = Seedling Length ×Germination Percent
5. Seedling Vigor Index II (Abdul Baki and Anderson, 1973)
Seedling Vigor Index II = Seedling Dry Weight×Germination Percent
Statistical analysis
The data obtained from the present experiments were subjected to statistical analysis through Randomized Block Design and the significance of each treatment was calculated as suggested by (Cochran and Cox, 1964). The generated data were analyzed through OPSTAT online analysis software developed by CCS Hisar Agriculture University, Haryana.
Effect of PGPR on Seed quality parameters of harvested seeds of Finger millet
As compared to control all seed quality parameters were significantly increased by the application of plant growthpromoting rhizobacteria (Bacillus amyloliquefaciens and Pseudomonas fluorescens) alone and in combination. Seed quality parameters (Table 1) were recorded in terms of test weight of seed, protein content, Nitrogen content, Phosphorus content and Potassium content of the seed.
| Treatment | 1000 seed weight (g) | Protein content (µg/mg) | Nitrogen content (%) | Phosphorus content (%) | Potassium content (%) |
|---|---|---|---|---|---|
| Control | 2.10 | 16.76 | 2.03 | 0.569 | 0.415 |
| BS-27 | 2.28 | 19.06 | 2.84 | 0.925 | 0.463 |
| BS-58 | 2.46 | 23.40 | 3.78 | 0.810 | 0.498 |
| Y-19 | 2.23 | 18.81 | 2.82 | 0.805 | 0.474 |
| BS-27+BS-58 | 2.33 | 18.49 | 3.71 | 0.669 | 0.462 |
| BS-58+Y-19 | 2.27 | 26.06 | 4.22 | 0.931 | 0.503 |
| BS-27+Y-19 | 2.22 | 19.96 | 3.03 | 0.688 | 0.452 |
| BS-58+BS-27+ Y-19 | 2.23 | 17.92 | 2.68 | 0.668 | 0.449 |
| C.D. | 0.080 | 0.373 | 0.027 | 0.005 | 0.002 |
| SE(m) | 0.026 | 0.122 | 0.009 | 0.002 | 0.001 |
| SE(d) | 0.037 | 0.172 | 0.012 | 0.002 | 0.001 |
| C.V. | 2.001 | 1.052 | 0.206 | 0.376 | 0.287 |
a. 1000 seed (test) weight of seeds
It is evident from Table 1 that all the treatments of PGPR resulted in increase test (1000 seed) weight (g). The 1000 seed weight, in general, ranged between 2.22 to 2.46 g in different treatments as compared to 2.10g in control. Amongst different treatments, a maximum 1000 seed weight of 2.46 g was recorded in T-2 (BS-58) followed by T-4 (BS-27 + BS-58) (2.33 g). The 1000 seed weight (2.10 g) in untreated control was observed minimum, which was statistically different from the rest of the treatment.
b. Protein content (μg/mg) of seed
It is evident from Table 1 that all the treatments of PGPR increased the protein content of seed (μg/mg). The protein content of the seed, in general, ranged between 17.92 to 26.06 μg/mg in different treatments as compared to 16.76 μg/mg in control. Amongst different treatments, the maximum protein content of seed 26.06 μg/mg was recorded in T-5 (BS-58+Y-19) followed by T-2 (BS-58) 23.40 μg/mg. The protein content (16.76 μg/mg) of seed in untreated control was observed minimum, which was statistically different from the rest of the treatment.
c. Nitrogen content (%) of seeds
The data on nitrogen content in the seed is presented in Table 1. The results indicate the significant effect of various treatments on Nitrogen content in seed. Significant and highest Nitrogen content in seed (4.22%) was recorded in T-5 (BS-58+Y-19) followed by 3.78% in T-2 (BS-58) while minimum nitrogen content in seed was recorded in control (2.03%)
d. Phosphorus content of seeds
The data on phosphorus content in the seed is presented in Table 1. The phosphorus content of seeds ranged between 0.668 from 0.931% in different treatments as compared to 0.569% in control. Amongst different treatments, maximum phosphorus content in the seed of 0.931% was recorded in T-5 (BS-58+Y-19 followed 0.925% by T-1 (BS-27). The phosphorus content of seeds 0.569% in untreated control was observed minimum, which was statistically different from the rest of the treatment.
e. Potassium content (%) in finger millet seed
The data on potassium content in the seed is presented in Table 1. The results indicate the significant effect of various treatments on potassium content in seed. The potassium content of seeds ranged between 0.449 from 0.505% in different treatments as compared to 0.415% in control. Amongst different treatments, maximum potassium content in the seed of 0.503% was recorded in T-5 (BS- 58+Y-19 followed 0.498% by T-2 (BS-58). The potassium content of seeds 0.415% in untreated control was observed minimum, which was statistically different from the rest of the treatment.
Effect of PGPR on seed germination and seedling growth of harvested seeds of finger millet
Seed germination and seedling growth are of prime importance in crop cultivation. For a healthy standing crop and higher productivity, it is important to raise healthy seedlings. The seed germination is affected by various external and internal factors. The application of PGPR in the present investigation significantly affected the seed germination parameters after harvesting of seed. The findings from the investigation regarding seed germination and various other seed germination traits are presented in Tables 2 and 3.
| Treatment | Germination % | Shoot length (cm) | Root length (cm) | Seedling length (cm) | Seedling fresh weight (mg) | Seedling dry weight (mg) |
|---|---|---|---|---|---|---|
| Control | 81.00 | 3.95 | 2.24 | 6.19 | 36.31 | 3.45 |
| BS-27 | 87.67 | 4.34 | 3.72 | 8.04 | 40.88 | 4.17 |
| BS-58 | 95.50 | 4.92 | 4.19 | 9.11 | 41.69 | 4.43 |
| Y-19 | 89.50 | 4.41 | 3.96 | 8.37 | 37.13 | 4.00 |
| BS-27+BS-58 | 88.50 | 4.55 | 3.09 | 7.64 | 40.88 | 4.10 |
| BS-58+Y-19 | 93.00 | 4.86 | 4.73 | 9.58 | 43.23 | 4.73 |
| BS-27+Y-19 | 87.00 | 4.52 | 3.26 | 7.79 | 39.78 | 3.62 |
| BS-58+BS-27+ Y-19 | 90.33 | 4.46 | 3.74 | 8.20 | 40.43 | 4.04 |
| C.D. | 1.921 | 0.027 | 0.025 | 0.018 | 0.044 | 0.024 |
| SE(m) | 0.627 | 0.009 | 0.008 | 0.006 | 0.014 | 0.008 |
| SE(d) | 0.887 | 0.013 | 0.011 | 0.008 | 0.020 | 0.011 |
| C.V. | 1.220 | 0.343 | 0.388 | 0.126 | 0.062 | 0.335 |
| Treatment | Speed of germination | Mean daily germination | Vigour-I | Vigour-II |
|---|---|---|---|---|
| Control | 57.84 | 10.13 | 501.39 | 279.45 |
| BS-27 | 65.73 | 10.94 | 704.87 | 365.58 |
| BS-58 | 59.27 | 11.94 | 870.01 | 423.07 |
| Y-19 | 63.45 | 11.19 | 749.12 | 358.00 |
| BS-27+BS-58 | 62.50 | 11.06 | 676.14 | 362.85 |
| BS-58+Y-19 | 61.89 | 11.63 | 890.94 | 439.89 |
| BS-27+Y-19 | 62.30 | 10.88 | 677.73 | 314.94 |
| BS-58+BS-27+ Y-19 | 62.36 | 11.29 | 740.71 | 364.93 |
| C.D. | 0.051 | 0.064 | 39.297 | 13.599 |
| SE(m) | 0.017 | 0.021 | 12.831 | 19.231 |
| SE(d) | 0.024 | 0.029 | 18.146 | 6.476 |
| C.V. | 0.047 | 0.323 | 3.060 |
a. Germination per cent
It is evident from Table 2 that all the treatments of PGPR resulted in increase seed germination percent. The seed germination percent, in general, ranged between 87 to 95.50% in different treatments as compared to 81% in control. Amongst different treatments, maximum seed germination of 95.50% was recorded in T-2 (BS-58) followed by T-5 (BS-58+Y-19) (93%). The seed germination percent in untreated control was observed minimum, which was statistically different from the rest of the treatment.
Seedling length (cm)
Seedling growth was significantly influenced in PGPR treated harvested seed. The shoot length was recorded a maximum of 4.92 cm in T-2 (BS-58) followed 4.86 by T-5 (BS- 58+Y-19), whereas root length was recorded highest 4.73 cm in T-5 (BS-58+Y-19) followed by 4.19 cm in T-2 (BS-58) and minimum shoot length (3.95 cm) and root length (2.24 cm) was recorded in control. The total length of the seedling was obtained by adding root and shoot length (cm).
The seedling length, in general, ranged from 7.64 to 9.58 cm in different treatments as compared to 6.19 cm in control. It was observed that T-5 (BS-58+Y-19) has the highest (9.58 cm) seedling length followed by T-2 (9.11 cm) which is significantly higher than that of other treatment and control.
Seedling fresh weight (mg)
Seedling fresh weight was significantly influenced by PGPR treatment in the harvested seed. The seedling fresh weight under PGPR treatment ranges from 37.13 to 43.23 mg in different treatments as compared to 36.31 mg in control. Maximum (43.23 mg) seedling fresh weight was recorded in T-5 (BS-58+Y-19) followed 41.69 mg by T-2 (BS-58) while minimum (36.31 mg) seedling fresh weight was recorded in control overall treatment.
Seedling dry weight (mg)
The seedling dry weight was significantly influenced by PGPR treatment compared to control. The seedling dry weight, in general, ranged between 3.62 to 4.73 mg different treatments as compared to control. The maximum (4.73 mg) seedling dry weight was recorded in T-5 (BS-58+Y-19) followed 4.43 mg by T-2 (BS-58). The seedling dry weight of 3.45 mg in untreated control was observed minimum, which was statistically different from the rest of the treatment.
Speed of germination (SG)
Data observed for the speed of germination shows that the rate of seed germination was significantly affected by the PGPR treatment. The speed of germination, in general, ranged from 59.27 to 65.73 in different treatments as compared to control. It was observed that the speed of germination is significantly highest (65.73) in T-1 (BS-27) followed (63.45) by T-3 (Y-19) and minimum (57.84) speed of germination was observed in control from rest of the treatment.
Mean Daily Germination (MDG)
Mean daily germination was observed highest (11.94) in T-2 (BS-58) followed by T-5 (BS-58+Y-19) (11.63) and lowest in control (10.13).
Seedling Vigour Index-I (SVI-I)
Data presented in Table 3 revealed that the Seed Vigour Index-I, in general range between 676.14 to 890.94 in different treatments as compared to 501.39 in control. Amongst different treatments, maximum seed vigour index-I of 890.94 was recorded in T5 (BS-58+Y-19) followed by 870.01 in T-2 (BS-58). The Seed vigour index-I in untreated control was recording minimum, which was statistically lower than the rest of the treatments.
Seedling Vigour Index-II (SVI-II)
Data presented in Table 3 revealed that the Seed Vigour Index-II, in general range between 314.94 to 439.89 in different treatments as compared to 279.45 in control. Amongst different treatments, maximum seed vigour index- II of 439.89 was recorded in T5 (BS-58+Y-19) followed by 423.07 in T-2 (BS-58). The Seed vigour index-II in untreated control was recording minimum, which was statistically lower than the rest of the treatments.
Seed treated with these bacterial strains Bacillus amyloliquefaciens (BS-58), and Pseudomonas fluorescens (Y-19) through in field condition, had provided sufficient positive results regarding the potential of these strains as plant growth-promoting (PGP) agents in finger millet crop. Inoculation with selected strains, BS-58 and Y-19 was found to enhance seed quality and seed germination of harvested seeds.
Effect of PGPR on Seed quality parameters of harvested seeds of Finger millet
It is evident from Table 1 that all the treatments of PGPR application alone and their combinations increased seed quality parameters i.e., 1000 seed weight (g), Protein content, Nitrogen content phosphorus content and potassium content of seed as compared to untreated control.
In the present study, 1000 seed weight was significant increase 17% by T-2 followed by T-5 11% as compared to control. The increase in 1000 seed weight indicates the production of bold seeds might because of higher nutrient availability and higher assimilation of nutrients in the plant. The growth substances are having a positive effect on processes like cell division and cell enlargement which are responsible for producing bolder seeds. The present results are in accordance with the findings of (Yadegari, Rahmani, 2010) studied that effect of co-inoculation with plant growthpromoting rhizobacteria (PGPR) and Rhizobium on yield components in common bean and found that treatment with PGPR significantly increased 100 seed weight (g).
The Protein content of the seed was influenced by PGPR treatments. The highest protein content 55% was increased by T-5 followed 40% by T-2. The increment of protein content was signifying the activity of plant growth-promoting bacteria especially due to the nitrogenfixing bacteria as they rapidly mobilization the nitrogen contentment of organic matter which is readily available to the plant. They also enhance the production of endogenous phytohormones (IAA and GA) which played an important role in forming a vigorous active root system. Thus enhances the nutrients assimilation, photosynthesis rate and translocation of solutes as well as accumulation of N within the seed. As reported in soybean that effective inoculation with Bradyrhizobium strains increased protein content up to 26%. The results obtained were in agreement with those recorded by (Zaki et al., 2012). Thus, the enhanced supply of nitrogen to the plant influenced the protein content, amino acids content, protoplasm and photosynthetic pigments (Chlorophyll). Apart from these, it influences the cell size, leaf area and photosynthetic activity (Azeez, 2009; Namvar et al., 2012; Daneshmand et al., 2012; Piccinin et al., 2013; Diacono et al., 2013).
The Nitrogen content of seed was reported to increase by 108% in T-5 followed by 86% in T-2 as compared to control. The results show a significant increase in nitrogen content with the application of biofertilizers. The increase in nitrogen content conforms to studies done by (Rana et al., 2012). It shows the nutrient mobilization activity of microorganism and reduces the dependency on chemical fertilizers, especially by using nitrogen-fixing and phosphorus solubilizing bacteria, which facilitate nitrogen and phosphorus uptake in plants (Cakmakci et al., 2007).
Moreover, the increase in nitrogen concentration may be due to inoculation of PGPR strains which show nitrate reductase activities and facilitate the uptake of ammonia and amino acids produced by plants (Prasanna et al.,2015). Similar results were obtained by (Kalibhavi et al., 2003; Duryodhana et al., 2004; Rathore et al., 2006; Choudhary and Gautam, 2007).The phosphorus content of seed was significantly increased by 64% in T-5 and 63% in T-1 as compared to control. The present investigation showed that using biofertilizers increased the phosphorus content of seeds as shown in Figure 1. The increased phosphorus content may be partially attributed to the production of the number of organic acids by the inoculated PGPR which reduces the soil pH, leads to the conversion of the nonavailable form of phosphorus into the available phosphorus. These results obtained were Similar findings were reported by (Dey et al., 2004) which shows that inoculation of phosphate solubilizing P. fluorescens has enhanced growth, yield, nitrogen and phosphorus concentrations in the seeds of peanut.
T1: BS-27, T2: BS-58, T3: Y-19, T4: BS-27+BS-58, T5: BS-58+Y-19, T6: BS-27+Y-19, T7: BS-27+ BS-58+Y-19.
The potassium content of the seed increased with the application of PGPR. It increased by 21% in T-5 and 20% in T-2 from control. The present research revealed that combined application of PGPR strains affects potassium content as compared to sole treatment indicating the supply of organic acids, macro and micronutrient due to improvement in the physical and biological health of the soil by increasing the water and nutrient holding capacity and aeration in soil by using PGPR.
Effect of PGPR on Seed germination and growth parameters of the harvested seeds of Finger millet
Seed germination and seedling growth are of prime importance in crop cultivation. For a healthy standing crop and higher productivity, it is important to raise healthy seedlings. Seed germination is affected by various factors. The application of PGPRs in the present investigation significantly affected the seed germination parameters after the harvest of seeds. The results obtained during the investigation regarding seed germination parameters are presented in Tables 2 and 3 and Figures 2 and 3.
T1: BS-27, T2: BS-58, T3: Y-19, T4: BS-27+BS-58, T5: BS-58+Y-19, T6: BS-27+Y-19, T7: BS-27+ BS-58+Y-19.
T1: BS-27, T2: BS-58, T3: Y-19, T4: BS-27+BS-58, T5: BS-58+Y-19, T6: BS-27+Y-19, T7: BS-27+ BS-58+Y-19.
The germination percent was significantly increased with the application of PGPR treatments. The maximum increase of germination percentage was recorded 18% in T-2 followed by 15% in T-5 (Figure 1). Values obtained shows the optimum availability of nutrients at all stages of plant growth and thus, produces bold, good quality and vigorous seeds resulting ultimately in enhanced germination. The present findings are in accordance with the findings of (Kanchana et al., 2014) who have reported that combined inoculation of PGPR recorded the highest germination of harvested seed in chilli. (Gupta et al., 2015) also reported increased germination of harvested seed in capsicum with the inoculation of PGPR also reported increased germination of harvested seed in barley with PGPR treatments. Similar results were reported by (Pandey et al., 2018) who observed a significant increase seed germination of Amaranths by Bacillus strain (BS-58).
An increase in seedling length by using PGPRs was observed in a range from 23% to 55% in harvested seed (Figure 2).
In the present study, recorded increased seedling length of 55% in T-5 followed by 47% in T-2 as compared to control. This may be due to the accumulation of storage food material which resulted in better seedling length. Similar findings were observed by (Bellishree, 2014) he reported that B. subtilis BCA-6 and B. pumilis BCA-19 enhanced root lengths also shoot lengths significantly in tomato. Vigour indexes of the seedlings define the quality of seedlings and help in predicting the survivability and quality of crop plants. (Pandey et al., 2018) also observed a significant increase of 58.12% in seedling growth of Amaranths by Bacillus (BS-58) treatment. The present findings are in agreement with the findings of (Gupta et al., 2015; Kanchana et al., 2014).
The seedling fresh and dry weight is presented in Table 3. In the present study, maximum seedling fresh and dry weight was recorded 19% and 37% respectively in T-5 followed by T-2. It might be due to more vigorous and healthy seedling produce by combined application of PGPR (Bacillus and Pseudomonas strains) that play important role in plant growth promotion agent. Similar results were reported by (Bellishree, 2014) that an increase in dry weight of shoot (1.13 g) root (0.08 g) was observed in B. subtilis Strain BCA- 6 in tomato. Similar to the present findings (Mandyal et al., 2012) also reported that bell pepper inoculated with a Bacillus isolate increased plant biomass and root biomass. (Pandey et al., 2018) also observed a significant increase in fresh weight (155%) and dry weight (85%) of Amaranths by Bacillus (BS-58) treatment.
The seed vigour index-I (SVI-I) depicted in Figure 3. The seedling Vigour-I was increased 78% in T-5 followed by 74% in T-2. The seedling Vigour-II was increased 57% in T-5 followed by 51% in T-2. Vigour indexes of the seedlings define the quality of seedlings and help in predicting the survivability and quality of crop plants the higher the values of SVI. This study showed the practical benefits of employing PGPR for a sustainable farming system. Similar findings were observed by (Pacome., 2013) in maize, seeds inoculated with the combination of P. fluorescens, P. putida. He reported the highest vigor index which was 77.54% higher in the combination than the control. Similar results were reported by (Pandey et al., 2018) who observed significantly increase vigour indices (117%, 151.94%) of Amaranths by Bacillus strain (BS-58). also reported increased seedling vigour indexes 30% to 60% in the harvested seed of barley with PGPR treatments.
In the present study, these PGPR treatments might have provided optimum availability of nutrients to the inoculated plants at all growth stages and thus gave bold, good quality and vigorous seeds resulting ultimately in higher germination. These results are in line with the findings of (Pacome, 2013; Naik et al., 2008; Kamal et al., 2015; Prathibha, Siddalingeshwara, 2013; Widawati, Suliasih,2018; Deepa et al.,2017; Pandey et al., 2018; Gupta et al., 2015; Kanchana et al., 2014; Mandyal et al., 2012).
This study clearly showed that the inoculation of finger millet plants with the bacterial strains Pseudomonas fluorescence (Y-19) and Bacillus amyloliquefaciens (BS-58) in combination had important effects on the Seed quality of finger millet seeds after harvest. PGPR strains significantly promoted seed quality i.e., 1000 seed weight, protein, nitrogen, phosphorus and potassium content of finger millet seed. PGPR strains alone Bs-58 and in a combination of BS-58 and Y-19 are the best treatments to increase the germination and seedling growth parameters of finger millet seed after harvest. So these PGPR can be used to improve the quality of finger millet. The combination is an easy thing to do with a relatively low cost to help eco-friendly farming in the future.