

Review Article - Journal of Research in Environmental Science and Toxicology ( 2025) Volume 14, Issue 1
Received: 15-Feb-2024, Manuscript No. irjass-24-127671; Editor assigned: 16-Feb-2024, Pre QC No. irjass-24-127671 (PQ); Reviewed: 04-Mar-2024, QC No. irjass-24-127671; Revised: 15-Apr-2025, Manuscript No. irjass-24-127671 (R); Published: 22-Apr-2025, DOI: 10.14303/2315-5698.2025.728
The starch was characterized using FTIR. The corrosion inhibition effectiveness was investigated using weight loss method. The results obtained show that cassava starch is a good inhibitor and the inhibitory efficiency of 88% is higher than that of millet starch starch 60.3%. the adsorption of both millet and cassava starch on steel surface obeys Temkin adsorption isotherm. This study revealed that both millet and cassava starch can be used as corrosion inhibition in acidic medium. However, the inhibition performance of millet starch is lower than that of cassa va starch. It is suggested that this difference in inhibition efficiency may be due to a difference in proportion of short amylopectin chain and amylose content. The isolated starch of cassava showed negligible iodine affinity indicating that the amylose c o ntent of starch was very low (<1.0%). Cassava starch can be a good for studying the influence of amylopectin fine structure on adsorption characteristic of starch
Millet, Cassava starch, Temkin adsorption isotherm, Weight loss method, Negligible iodine
Background to the study
Corrosion is a natural process that converts refined metal into a more chemically stable form like sulfide, oxide or hydroxide (Charitha, et al., 2017). It is the gradual destruction of materials and its properties especially metals by electrochemical or chemical reaction with their environment (Nwanonenyi, et al., 2016).
Corrosion is one of the most challenging factors facing petrochemical and petroleum industries. It causes loss of wealth and affects daily life welfare (Dudukcu, et al., 2020).
Metallic deterioration proceeds at high rate after the destruction the passive barricade which is caused by many of reactions that change the composition and behavior of both the metal surface and the environment (Erna, et al., 2019). This is observed in, for instance, oxides formation, metal cation diffusion into the coating matrix, local pH changes, and electrochemical potential (Kermani, et al., 1996).
In the petrochemical, transportation, oil and gas, and automobile industries metallic corrosion is one of the major factors affecting the dependability of the systems (Sharma, et al., 2019).
For example, in most petrochemical industries, about thousands of kilometers of pipeline, pressure, pumps and storage vessels are used to store, transport and process products (Ochoa, et al., 2013). This frame is not only for development of the industry but also to the national economy. Therefore, carbon steel is the major metal used in the installation and inevitably to corrosion (Nwanonenyi, et al., 2016).
Corrosion is unpreventable phenomena but it can be eliminated or controlled using inhibitors (Inhibitors are compounds that are used to protect and prevent metal from corrosion particularly in acidic medium (Nwanonenyi, et al., 2016). It is also slow down the rate of corrosion).
There are two types of inhibitors; chemical and inorganic inhibitors. Most chemical inhibitors are non-biodegradable; such as, chromate, silicate, carbonate and so, on (Sivakumar, et al., 2020). Theses chemical inhibitors are fairly inexpensive but cause more harm than the solution they provided (Aejitha, et al., 2016).
Example of organic inhibitor: herbal plants, gum Arabic, yam, millet starch and so, on. Starch is viewed as ecologically friendly and economically suitable inhibitors (Li et al., 2020). Starch is physical and natural and biodegradable polymer which is relatively inexpensive and readily available in our environment (Falua, et al., 2022).
Starch is carbohydrate (polysaccharide) composed of heavier compound of glucose units connected together by glyosidic bond (is a type of covalent bond that link a carbohydrate molecule to another with may or may not be another sugar group). The two components of starch are amylopectin (80%-85%) and amylose (15%-20%) (Robertson, et al., 2006).
Starch is used in different fields such as, pharmaceutical, textile industries, paper making industries and food processing industries. Many scientists in their literature review figured out important of using starch in controlling corrosion of carbon steel and alloy steel.
Carbon steel is kind of carbon steel with low amount of carbon. It is commonly known as low carbon steel (Palanisamy, et al., 2020). The amount of carbon is ranged 0.05% to 0.25% by mass where as high carbon steel having carbon content around 0.30% to 2.0% (Figure 1).
Figure 1. Structure of amylose and amylopectin.
Problem statement
Corrosion is one of the major factors responsible for negative impact on capital, operational expenditure, safety health, environmental and economic damages. Corrosion is an environmental process prevailing in petrochemical industries. Impact of corrosion on economic, safety is one of the tremendous challenging in petrochemical industries. Subsequently, production environment seems to be more corrosive hence demanding a realistic corrosion management. It is stated that over 25% of failures experienced in petrochemical industries is related with corrosion. 50% of these failures are related with sweet and sour producing fluid such as CO2 and H2S.
Aim of the research
• The aim of this research is to determine and compare the potential and effectiveness of starch isolated from cereal grain and tubers as corrosion inhibitors.
• It relates to the making of measurable data and testable experiment towards the control or elimination of corrosion induced for operational expenditure, health, environmental and economic damages through eco-friendly inhibitors.
Specific objectives of the study
• To compare the potential and effectiveness of starch isolated from cereal grain and tubers as corrosion inhibitors.
• Structural characterization of the starches using FTIR (Fourier Transformation Infrared Spectroscopy).
• Relationship of starch structure from different sources to corrosion efficiency.
• Corrosion inhibition evaluation using weight loss measurement.
• To determine metal-inhibitor interaction mechanism using adsorption isotherms.
This focuses on works done by many scholars relevant to corrosion of carbon, aluminum and mild steel in different environment with different concentration of organic and inorganic inhibitors. Many environmental conditions occurred in industries and many metals came across to this condition.
The corrosion inhibition characteristics of biopolymer starch was studied as an eco-friendly inhibitor for the corrosion control of 6061 aluminum alloy in 0.1M HCl.
Electrochemical methods such as Potentiodynamic Polarization (PDP) and Electrochemical Impedance Spectroscopy (EIS) techniques were adopted. Effect of inhibitor concentrations (in the range of 100 to 800 ppm) was studied at temperatures of 30, 35, 40, 45 and 50â??. The kinetic and thermodynamic parameters for corrosion and inhibition process were determined. The surface morphology of aluminum in the absence and presence of starch in 0.1 M HCl solution was studied using Scanning Electron Microscope (SEM) and Electron Dispersive X-ray spectroscopy (EDX). Suitable mechanism was proposed for corrosion and inhibition process. The percentage inhibition efficiency of starch increased with increasing inhibitor concentrations and also with increase in temperature. Starch acted as a mixed inhibitor, underwent chemical adsorption and obeyed Langmuir adsorption isotherm. Starch–a biodegradable polymer emerged as a potential, cost effective environmentally begin inhibitor for the corrosion control of 6061-aluminum in HCl.
Subhaian et al., in their research “comparative study of corrosion inhibition of commiphora caudata and digera muricata for mild steel in 1 M HCl Solution” shows that commiphora caudata and digera muricata extracts as corrosion inhibitors for mild steel in 1 M HCl were studied by weight loss and surface analysis techniques. It showed that both plant extracts inhibited the mild steel corrosion. Inhibition efficiency increased with increasing inhibitor concentration and temperature which suggested that the chemical mechanism. The inhibitors adsorbed onto the mild steel surface followed Langmuir adsorption isotherm. The surface morphology of mild steel showed that the addition of the extract reduces the mild steel corrosion.
Millet Starch (MS) was extracted from millet grains and modified using a physical method (pregelatinization). The corrosion inhibition effectiveness of millet starch on mild steel corrosion in 0.5 M H2SO4 solution was investigated using weight loss measurement, potentiodynamic polarization and chemical quantum methods. The results obtained show that pure millet starch and its combination with potassium iodide effectively reduced the corrosion of mild steel in 0.5 M H2SO4 solution with an inhibition efficiency of 87.14% and 94.03% respectively. The increase in inhibition on addition of potassium iodide showed synergistic effect. In addition, millet starch was found to function essentially as a mixed-type inhibitor by adsorption on the mild steel surface according to the polarization curves. The mode of inhibition adsorption was best modeled using Langmuir adsorption isotherm. The calculated values of ΔGads, Ea and ΔHads suggested physisorption mechanism. Theoretical quantum chemical calculations were performed to confirm the ability of starch to adsorb onto mild steel surface.
Native starch extracted from sweet potato tubers was modified via extrusion. The native starch and modified starch were characterized by Fourier Transform Infrared (FTIR) spectroscopy, which revealed slight modifications in peak position and intensities on starch modification.
The modified starch was evaluated as a corrosion inhibitor of galvanized steel in 1 M HCl solution by gravimetric and potentiodynamic polarization measurement techniques. Results obtained from gravimetric measurement reveals that the modified starch inhibition efficacy was dependent on time, concentration and temperature, increasing with increase in concentration and decreasing with increase in time of immersion and temperature of the system. Maximum inhibition efficiency of 64.26% was obtained at a concentration of 0.7 g/L PMS. Also, result from polarization technique indicated that the modified starch belonged to a mixed-type inhibitor. Adsorption of the inhibitor on the galvanized steel surface was found to obey the Langmuir adsorption isotherm.
The corrosion inhibition of aluminum in 2 M H2SO4 by millet starch was investigated using gravimetric technique by Nwanonenyi et al., at 35-65°C and theoretical quantum chemical computations. The results indicated that millet starch functioned as a good inhibitor for acid induced corrosion of aluminum. It was found that increase in inhibition efficiency of the inhibitor was concentration dependent and also addition of potassium iodide increased inhibitive performance of the inhibitor synergistically. Furthermore, the mode of adsorption process of the inhibitor was best modeled using Langmuir adsorption isotherm at all inhibitor concentrations and temperatures studied. The trend of inhibition efficiency with temperature, calculated values of free energy, activation energy and enthalpy of adsorption was used to propose the inhibition mechanism. Theoretical chemical quantum computations were carried out using density functional theory to underscore the relationship existing between the inhibitive performance of millet starch and electronic properties of millet starch. Finally, molecular dynamic simulations were performed using Forcite quench molecular dynamics to model lowest energy adsorption configurations of the starch molecule on Al surface and to determine the binding energy of adsorption.
Activated (AS) and carboxymethylated (CMS0.24) cassava starch derivatives were studied as corrosion inhibitors for carbon steel XC35 in a 200 mgL–1 NaCl solution. They were characterized by back titration and Fourier Transform Infrared Spectroscopy (FTIR). Electrochemical techniques were used to evaluate the inhibitive properties of starches at room temperature and the chemical composition of the protective films was determined by X-Ray Photoelectron Spectroscopy (XPS). Electrochemical measurements revealed that AS acts as mixed inhibitor, whereas CMS0.24 mainly inhibits the anodic reaction. In both cases, the protection increased with the inhibitor concentration; nevertheless, after 24 hours of immersion, the CMS0.24 loses its properties, while AS molecules still maintains them. XPS analyses show that the inhibitive films are composed of an iron oxide/hydroxide mixture in which starch molecules are incorporated. Results were explained taking into consideration the hydrophilicity and the strength of the ionic interaction of the starches with the metal surface.
Cassava starch ternary graft copolymer of Cassava Starch-Sodium Allylsulfonate-Acryl Amide Graft Copolymer (CS-SAS-AAGC) was synthesized through Cassava Starch (CS) chemically modified by grafting two monomers of Sodium Allylsulfonate (SAS) and Acryl Amide (AA) simultaneously. The inhibition performance of CS-SAS-AAGC on Cold Rolled Steel (CRS) in 1.0 M HCl solution was experimentally studied by weight loss, electrochemical techniques and surface analysis, and its adsorption was theoretically investigated by both quantum chemical calculation and Molecular Dynamic (MD) simulation. The results indicate that CS-SAS-AAGC shows an optimum inhibition efficiency as high as 97.2% at 50 mg L−1, and its inhibitive ability exhibits stronger than that of CS, SAS or AA. CS-SAS-AAGC behaves as a mixed-type inhibitor through geometric blocking effect. SEM and AFM images clearly reveal that the corrosion of CRS surface is efficiently retarded by CS-SAS-AAGC. Contact angle result suggests that the inhibited CRS surface is of hydrophobic nature. The adsorption active sites of CS-SAS-AAGC are mainly the grafted monomers of SAS and AA.
A comparative study on the inhibitory effect of various parts of the plant MimusapsElangi (ME) extract (leaves, fruits, barks, seeds) on the corrosion of mild steel in 1 N HCl medium was investigated by Sivakumar. Using weight loss method, potentiodynamic polarization and electrochemical impedance spectroscopy techniques. The polarization studies revealed that the plants extract act as mixed type inhibitor. It was found from the weight loss method that the inhibition efficiency of ME extracts increases in concentration dependents manner which was also supported by the results of electrochemical techniques. On comparison, maximum inhibition efficiency was found in ME leaves extracts with 98.50% at 20 ppm concentration. The SEM morphology of the adsorbed protective film on the mild steel surface has confirmed the high performance of inhibitive effect of the plant extract. Surface coverage values were tested graphically for suitable adsorption. Temperature studies revealed decrease in inhibition efficiency with increase temperature which suggests physisorption mechanism.
Materials
• Yam grain
• Cassava grain
• Carbon steel
• Hydrochloric acid
• Beakers
• Mortar and pestle
• Analytical weighing balance
• Infrared (IR) spectrometer.
• Distilled water
Starch extraction
• 453 grams of cassava and 452 grams of millet grain were weighted and then underwent the following separation processes.
• Washing (to removing excess brans)
• Decantation
• Filtration (settling)
• Purification of starch with distilled water.
• Drying
Finally, 360 grams of millet starch and 388 grams of cassava were obtained after underwent these processes.
Characterization of Starch Using Fourier Transformation Infrared spectroscopy (FTIR)
Both cassava and millet starch were characterized using FTIR.
FTIR offers quantitative and qualitative analysis for organic and inorganic samples. Fourier Transform Infrared Spectroscopy identifies chemical bonds in a molecule by producing an infrared absorption spectrum. FTIR is an effective analytical instrument for detecting functional groups and characterizing covalent bonding information (Mathias).
•About 2 mg of starch were mixed with KBR pellet and grinded with aid of mortal and pastel.
• The sample were put in the test tube and then inserted in IR plate.
• The results were obtained with good spectrum.
Preparation of carbon steel coupon
Metal used in this study is carbon steel. Carbon steels are series of alloys of carbon and iron containing up to about 1% carbon and up to 1.65% Mn, with elements added in specific quantities for deoxidization and residual quantities of other elements. Carbon steel is almost exclusive choice of pipeline designers. This is true for pipeline systems that are used to gather or collect the natural gas, crude oil, or water. Carbon steel was used because of its versatility in construction. It is mostly used in many petrochemical industries because of its strength and availability.
Carbon steel specimen was cut out to get rectangular surface with dimension of 7 mm wide and 6 cm long (Figure 2).
Figure 2. Carbon steel.
Preparation of acidic medium
0.1 M of HCl was prepared by diluting 1620 μL (1.6 mL of HCl) in 200 mL of distilled water.
Mathematically expression.
Specific gravity of HCl is 1.87 and its percentage is 37.5%
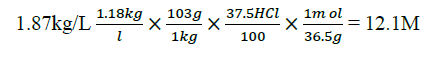
C1C2=V1V2
V2=0.1 M × 200 Ml ÷ 12.1 M
V2=1.62 mL
1.62 mL of HCl were diluted in 200 mL of distilled water to yield 0.1 M of HCl.
Set up for weight loss measurement
Weight loss measurement involves the weighing of coupon or sample of carbon steel before immersion in acidic medium with inhibitors and without inhibitors and after, and then reweighted after certain period of time. For instance, in this experiment, it run at 2 days’ interval for 16 days, this implied that every two days the coupons were removed, reweighted and re-immersed until the end of 16 days, explained in literature review (OMOTOSHO).
This section presents the result and discussion concerning characterization of starch, weight and weight loss, corrosion rate, surface coverage, inhibition efficiency and adsorption isotherms (Figure 3) (Tables 1 and 2).
Characterization of starch
The result obtained from FTIR Infrared spectroscopy was used to determine the functional group of each starches.
It found out in the IR spectra analysis, millet starch showed strong band in the region of carboxylic acid, amide and Sulphur.
Figure 3. Graph representation of FTIR.
| Filename | Peak at | Peak height |
| millet starch 2.spc [0] | 707.9 Amine NH2NH | 5.6 |
| millet starch 2.spc [0] | 814.7 alkenes C-C | 8.3 |
| millet starch 2.spc [0] | 1008.8 anhydride and alcohol | 1.1 |
| millet starch 2.spc [0] | 1150.4 carboxylic acid 0-H | 2.8 |
| millet starch 2.spc [0] | 1363. Sulphur S=o | 5 |
| millet starch 2.spc [0] | 1445.1 Aromatic C=C | 5.4 |
| millet starch 2.spc [0] | 1532.1 | 8 |
| millet starch 2.spc [0] | 1651.2 | 5.2 |
| millet starch 2.spc [0] | 1900.8 | 10.4 |
| millet starch 2.spc [0] | 2356.2 alkane CH | 4.6 |
| millet starch 2.spc [0] | 2460.9 | 6.6 |
| millet starch 2.spc [0] | 2923.4 | 1.6 |
| millet starch 2.spc [0] | 3298.4 | 0.5 |
| millet starch 2.spc [0] | 3593.7 | 1.1 |
| millet starch 2.spc [0] | 3696.1 alcohol OH stretch | 1.8 |
| millet starch 2.spc [0] | 3821.9 | 2.3 |
Table 1. Peak height calculations of millet starch.
| Filename | Peak at | Peak height |
| Cassava starch.spc [0] | 703.7 Amine N-H | 4.2 |
| Cassava starch.spc [0] | 819.4 | 8.5 |
| Cassava starch.spc [0] | 1048.0 Anhydride | 0.5 |
| Cassava starch.spc [0] | 1153.5 carboxylic acid | 1.3 |
| Cassava starch.spc [0] | 1361.5 Sulphur S=0 | 3.8 |
| Cassava starch.spc [0] | 1413.9 | 3.9 |
| Cassava starch.spc [0] | 1646.5 | 6.1 |
| Cassava starch.spc [0] | 1897.6 | 14.3 |
| Cassava starch.spc [0] | 2335.2 alkane CH | 11.4 |
| Cassava starch.spc [0] | 2925.1 | 1.8 |
| Cassava starch.spc [0] | 3506.5 Ketone C=o | 0.4 |
| Cassava starch.spc [0] | 3727.8 alcohol 0-H | 3.4 |
| Cassava starch.spc [0] | 3819.4 | 4.2 |
Table 2. Peak height calculations of cassava starch.
It found out in the IR spectra analysis, millet starch showed strong band in the region of carboxylic acid, amide and Sulphur.
FTIR spectrum of the starches illustrated that the O-H stretch peak at 3493.24 cm−1 is from the starch molecules. The peaks at 1745.65 cm−1 to 1236.42 cm−1 represent a carbonyl (COOH) group.
Weight loss measurement
This was done on carbon steel specimen immersed in 200 mL of test solution contained in a beaker and then kept at room temperature. The specimens were retrieved at 2 days’ interval progressively for 16 days. During stipulation immersion time the specimens were recovered from the solution immersed in acetone solution to remove corrosive particles that overlie on the surface of carbon steel or prevent further corrosion reaction, and then washed with distilled water, dried and reweighted mass loss. The weight loss was calculated using this formula by taking the difference between the final weight and initial weight at a given time (Table 3).
The values recorded in the table were average values of triplicated determinations (Figures 4 and 5).
Weight loss was calculated using this formula

W2-W1=Δw (Equation 1)
W2=Final weight of the coupons after immersion
W1=Initial weight before immersion
Δw=change in weight (weight loss).
| System mg/50 mL Cassava | 2 | 4 | 6 | 8 |
| Blank | 0.069 | 0.0458 | 0.0497 | 0.1455 |
| 7.5 | 0.001 | 0.016 | 0.037 | 0.037 |
| 15 | 0.0042 | 0.0182 | 0.0152 | 0.0355 |
| 22.5 | 0.0007 | 0.0024 | 0.0035 | 0.0049 |
| Millet | 2 | 4 | 6 | 8 |
| 7.5 | 0.098 | 0.06 | 0.075 | 0.0088 |
| 15 | 0.0207 | 0.0207 | 0.0207 | 0.0792 |
| 22.5 | 0.0029 | 0.0007 | 0.0011 | 0.0031 |
Table 3. Weight loss.
Figure 4. The graph of weight loss against time (days) of millet starch.
Figure 5. The graph of weight loss against time (days) of cassava starch.
The above table shows the weight loss of carbon steel in the absence and presence of different concentration of millet and cassava starch extract in 0.1 M hydrochloric acid at different time interval. The table obviously shows a reduction in weight loss of the coupons in the presence of these inhibitors compared to the blank solution.
Corrosion rate
The corrosion rate for carbon steel coupons in 0.1 M of HCl in the absence and presence of both millet and cassava starch of different immersion period of time are shown in the below table (Figure 6).
The formula used:
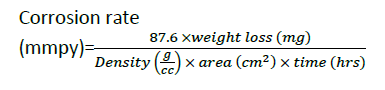
(Equation 2)
1mpy=0.0254 mm/y
where, ΔW=weight loss in gram(g), ρ=density of the metal coupons (g/cm3), A=exposed surface area of the metal coupon(cm2) and t=time of exposure (in hrs) (Table 4).
| System mg/50 mL Cassava | 2 | 4 | 6 | 8 |
| Blank | 0.00038 | 0.00126 | 0.00068 | 0.001 |
| 7.5 | 0.000053 | 8.85E-05 | 0.00051 | 0.00025 |
| 15 | 0.000041 | 0.000501 | 0.00021 | 0.00024 |
| 22.5 | 0.000038 | 0.000066 | 0.000048 | 0.000033 |
| Millet | 0.00038 | 0.00126 | 0.00068 | 0.001 |
| 7.5 | 0.000281 | 0.00061 | 0.00063 | 0.00075 |
| 15 | 0.00014 | 0.00057 | 0.00049 | 0.00063 |
| 22.5 | 0.000029 | 0.000059 | 0.000015 | 0.00021 |
Table 4. Inhibition efficiency and surface coverage.
Figure 6. Graph of corrosion rate against time of Cassava starch.
To interpretation the corrosion rate against time graph, the graph curves were considered, it was good to developed inequality table. This was done by averaging the corrosion rate value for each inhibitory concentration over 16 days.
The above graph shows the corrosion rate of carbon steel coupon was lower in the presence of both millet and cassava starch compared to the acidic medium without inhibitor. The corrosion rate decreases as the concentration of each starch increase, showing that the corrosion rate is depended on the amount of both millet and cassava starch. The graph showed that both millet and cassava inhibited the corrosion rate of carbon steel in 0.1 M of hydrochloric acid.
Therefore, the corrosion rate decreases with decrease in exposure time for both blank and inhibited solution,
Though, the corrosion rate of the starches were dropped drastically and decrease between day 6 but later increase at day 8.
This happened due to the temperature change or due to formation of discontinuous passive film on the surface of carbon steel as result of lower concentration in of starch in acidic medium.
The corrosion rate decreases with increased of time from day 2 to day 8 possibly as result of faster rate of adsorption.
Inhibitor efficiency
Inhibition efficiency and surface coverage of carbon steel in different concentration of millet and cassava starch in 0.1 M of hydrochloric acid at various time of immersion.
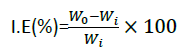
where, W0 and Wi blank are corrosion rates in the presence and absence of inhibitor respectively (Table 5).
| Inhibition efficiency % | 2 | 4 | 6 | 8 | Surface coverage | 2 | 4 | 6 | 8 |
| Millet starch | |||||||||
| Blank | ---- | === | ------ | ------ | ---- | === | ==== | ==== | ==== |
| 7.5 mg/50 ml | 86 | 29 | 25 | 75 | 0.86 | 0.29 | 0.25 | 0.75 | |
| 15 mg/50 ml | 89 | 60 | 69 | 76 | 0.89 | 0.6 | 0.69 | 0.89 | |
| 22.5 mg/50 ml | 90 | 94 | 92 | 96 | 0.9 | 0.94 | 0.92 | 0.96 | |
| Cassava starch | |||||||||
| 7.5 mg/50 ml | 26 | 51 | 7 | 25 | 0.26 | 0.51 | 0.7 | 0.25 | |
| 15 mg/50 ml | 63 | 54 | 27 | 27 | 0.63 | 0.54 | 0.27 | 0.27 | |
| 22.5 mg/50 ml | 92 | 95 | 97 | 79 | 0.92 | 0.95 | 0.97 | 0.79 |
Table 5. Inhibition efficiency and surface coverage of carbon steel in different concentration.
Based on this, it was possible to compare corrosion rate with inhibitory concentration since there is indirect relationship existence. We can see from the table, the higher the inhibitory concentration the lower the corrosion rate with higher inhibitory efficiency while the inhibitor concentration with higher corrosion rate had the lowest Inhibitory efficiency.
Adsorption isotherm
It describes the mechanism of inhibition reaction or the chemisorption process, when ionic covalent bond is made between adsorbate and adsorbent. The interaction between metal and inhibitors. The efficiency of corrosion inhibition is depended on its capability to adsorb on metal surface. The Langmuir was found to be the best for comparing the inhibition of millet and cassava starch in 0.1 M of HCl acid.
Langmuir adsorption isotherm
The Langmuir adsorption isotherm is used to describe the equilibrium between adsorbate and adsorbent system, where the adsorbate adsorption is limited to one molecular layer at or before a relative pressure of unity is reached (Lingling Liu and Sheng-Lian Luo).
Formula used
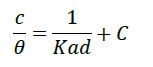
where θ=degree of surface coverage, C=inhibitor concentration, Kads=equilibrium constant of adsorption process (Table 6).
| System | 2 | 4 | 6 | 8 |
| Millet | ||||
| Blank | ----- | ----- | ---- | ---- |
| 7.5 mg/50 ml | 8.71 | 26 | 32.5 | 16 |
| 15 mg/50 ml | 16.8 | 25 | 22 | 23 |
| 22.5 mg/50 ml | 25 | 23 | 24 | 32 |
| cassava | ||||
| 7.5 mg/50 ml | 27.5 | 14.7 | 39.8 | 32 |
| 15 mg/50 ml | 23 | 27 | 55 | 55 |
| 22.5 mg/50 ml | 24.5 | 23.68 | 23.19 | 28.4 |
Table 6. Langmuir adsorption isotherm C/θ.
The experimental values were illustrated graphically with the best result for Langmuir adsorption isotherm. The graph of C/O Vs. concentration of adsorption isotherm is calculated by using formula (Figures 7 and 8).
Langmuir adsorption isotherm plot of log C/θ against concentration for hydrochloric acid. The graph of correlation coefficient R2 of Langmuir adsorption isotherm (Table 7).
Figure 7. Graph of Langmuir adsorption for cassava starch.
Figure 8. Graph of Langmuir adsorption for millet starch.
| Days | 2 | 4 | 6 | 8 |
| Correlation coefficient of cassava Starch | 0.997 | 0.5015 | 0.3285 | 0.9667 |
| Correlation coefficient millet Starch | 0.7943 | 0.4967 | 0.2156 | 0.3749 |
Table 7. Correlation coefficient of cassava starch and millet starch.
Temkin adsorption isotherm
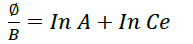
Where B=RT/b constant related to heat of sorption (J/mol) obtained from Temkin plot (qe versus in Ce, A intercept=Temkin isotherm equilibrium binding constant, b (slope)=Temkin isotherm constant, R=universal gas T=absolute temperature (Figures 9,10 & Table 8).
Figure 9. Graph of Temkin adsorption isotherm of millet starch.
Figure 10. Graph of Temkin adsorption isotherm of cassava starch.
| Days | 2 | 4 | 6 | 8 |
| Correlation coefficient of Cassava | 0.9814 | 0.971 | 0.9988 | 0.9979 |
| Correlation coefficient of Millet | 0.9945 | 0.8009 | 0.89 | 0.6478 |
Table 8. Table of correlation coefficient R2 of Temkin adsorption isotherm.
The study shows that, the increase in starch inhibition efficiency by both cassava and millet with increase in concentration is dealt with adsorption of cassava and millet on the surface of carbon steel.
The graph illustrated that, both cassava and millet starch adsorbed on the surface of carbon steel, the higher the concentration of starch attributing to the greater surface coverage to various adsorption isotherm.
It stated that the adsorption isotherm has best fit when correlation co-efficient is one or close to one (R2).
From graph I can say both millet and cassava starch obey Temkin adsorption isotherm because their values of R2 are closed to unity. This is implied that millet starch is more effective on the surface of the carbon steel.
Mechanism of inhibition
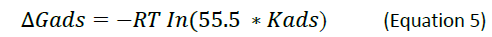
is the free energy of adsorption), where Kads represents the equilibrium constant of the adsorption process and C represents the inhibitor’s concentration(s), R represents the gas constant, while T represents the absolute temperature.
According to Maria Erna in her articles, more negative of free energy of adsorption indicates Chemo-adsorption whereas more positive values indicate physio- adsorption (Erna).
Mechanism of inhibition
Fe + Starch → Fe (Starch)ads
Fe (Starch)ads → Fe2+ + Starch + ne-
Starch + H2Oads → Starchads + H2Oaq
The chemisorption of starch on carbon steel is denoted by donor acceptor interaction between lone pair of electrons from carbonyl and amine group of the starch with d-orbital of Fe. Since the value of free energy in each starch has more negative, this confirm that the adsorption mechanism of starch on the surface of carbon steel was chemically adsorbed (Erna).
On the basis of this study, the following conclusion can be drawn.
The corrosion inhibition of millet and cassava starch on carbon steel was studied comparatively. Starch was extracted from millet and cassava, characterized using FTIR. Inhibition efficiency of cassava and millet 88% and 69.3% respectively. The adsorption of both millet and cassava starch obeys Temkin adsorption isotherm model. Corrosion efficiency of cassava is higher than millet starch. This could be explained on the basis of difference in amylopectin and amylose proportion.
• Weight loss measurement should be carried out for more than 16 days.
• Many adsorption isotherm models could be investigated.
• Other eco-friendly inhibitors could be investigated.
All thanks and glory be to Allah lord of the world, for sparing my life to make this report. My sincere appreciation goes to my supervisor Prof. Phillip Shallsuku for his effort to guide me and ensure the success and achievement of the project. My appreciation also goes to the Dr, Bolade Agboola, who also has made numerous sacrifices of his valuable time to help, developed and turned-out me to knob any challenges during my research. I would like to thank Dr. Victor Adams for her countless sacrifices of her precious time to help me. I would also like to thank Mr. Mohammed Falalu Yahaya for the guidance and suggestions during the experiment.
Last but not least, I would like to thank my entire family and friends especially Auwalu Sani Gambo, Shuaibu Sani Abubakar, Isa Sani Abubakar, Usman, Hamza , Aliyu Sani Abubakar, Nura, Rabiu, Muhammadu,Yakubu, Jazuli Magaji Ali Musa Suleman, Naziru Yunusa, Sani Abdullahi, Abubakar Habib, Bazallahi Muhammad Dahiru, Nura Ahamad, Ali Officer, Ahamad Jazuli, Muqaddas Jazuli, Umar Rabiu Biye, Kubra saadu, Rahmatu saidu, Usman Salihu, Maryam Mansur, Nazifi Abdulhadi, Asamau Shehu Maddibo Fatima Rabiu Adam and so,on.
My countless and sincere appreciation goes to my beloved Mum, Umma Musa Sule, hajiya Umma, Hajiya Amina, Hajiya Hafsatu and my beloved Father Alhaji Sani Mai atamfa (Lagos) May Aljannah Firdausi be your final destination. Thank you so much I love you all with all my heart.
I dedicate this project to the most gracious and the most merciful God and also to my lovely family.