

Research Article - International Research Journal of Basic and Clinical Studies ( 2023) Volume 8, Issue 4
Received: 01-Aug-2023, Manuscript No. irjbcs-23-110244; Editor assigned: 04-Aug-2023, Pre QC No. irjbcs-23-110244; Reviewed: 18-Aug-2023, QC No. irjbcs-23-110244; Revised: 25-Aug-2023, Manuscript No. irjbcs-23-110244; Published: 31-Aug-2023, DOI: 10.14303/irjbcs.2023.47
Background: The burden of type 2 diabetes mellitus(T2DM) is increasing worldwide and. It is a leading cause. Due to lack of funds and poor healthcare system, Sub-Saharan Africa is the worst affected with Nigeria bearing the greatest burden for the region. A major factor underlying the development of complication in type 2 diabetes is poor glycemic control and coagulation activation. Objective: To determine the impact of gender on glycemic and coagulation control in type 2 diabetes mellitus. Materials and Methods: This was an observational cross sectional study involving 100 type 2 diabetic mellitus patients (50 males and 50 females) aged 40-80 years. The activated partial thromboplastin time, prothrombin time, and the International normalized ratio were determined by manual method using kits obtained from Fortress Diagnostic Ltd, UK. The fasting blood sugar and the two hour post prandial glucose level were determined by the glucose oxidase enzymatic method with commercial kits obtained from Biologo Co. Ltd, France while the glycated hemoglobin levels were determined by the Ion exchange chromatography method with reagents obtained from Fortress Diagnostic kits, UK. The fibrinogen, von willebrand factor antigen and D-dimer levels were determined by ELISA with kits obtained from Shanghai Huirui Chemical Technology Co. Ltd, China. Data was analyzed using SPSS version 20 (IBM Statistics, Armok, USA). Differences were determined by Student Test and One Way Analysis of Variance. Result were presented as mean + SD. and P < 0.05 was considered significant. Results: The glycated hemoglobin and von willebrand factor levels were significantly higher for the female subjects (11.33±2.20 and 28.27±5.43) compared to the males (7.21±2.26 and 17.77±4.62) (p=0.031 and 0.002 respectively). The activated partial thromboplastin time was significantly lower in the female subjects (32.46±3.41) compared to the males (38.91±2.23) (p=0.003). Conclusion: This findings suggest that females with T2DM have poorer glycemic and coagulation control compared to males.
Glycemic control, Coagulation activation, Gender, Type 2 diabetes mellitus
Diabetes mellitus is a group of metabolic disorders characterized by abnormal carbohydrate metabolism resulting in chronic hyperglycemia caused by defective insulin production, action or both(Adana T, 2021) (Arkew M, 2021).Type 2 Diabetes mellitus (T2DM) is the most prevalent type of diabetes and accounts for about 90-95% of diabetes cases.( Narjis M, 2021) (Ali MH, 2019) It’s global prevalence has increased from 4.7% (108 million) in 1980 to 9.3% (463 million) in 2019 and postulated to increase to 10.2% (578 million) by 2030 as well as 10.9% (700 million) by 2045 as well.( Kaur R, 2018) (Park JM, 2021) It is also estimated that 15.5% (9.8-27.8 million) people in the Sub- Saharan Africa have type 2 diabetes with Nigeria having the highest burden of cases(Wang Y, 2021) (Zimmermann M, 2018). As type 2 Diabetes mellitus is emerging as a modern epidemic, studies of T2DM are also ever increasing to predict its risk and causative factors to improve the prognosis and treatment outcomes (Gupta G, 2019).
Gender have become a major concern in healthcare in recent years particularly in the field of metabolic and chronic diseases such as diabetes mellitus (Tramant B, 2023). It is a risk factor known to modify the course of many diseases such as the initiation, progression and the outcome of such diseases (Karuveetti V, 2022).Coagulation activation and poor glycemic control have been reported as the major events leading to the development of complications in type 2 diabetes mellitus(Groot HE, 2020).Development of risk assessment for treatment of type 2 diabetes mellitus can be better established by understand the effect of gender on the risk factors for development of complication such as coagulation activation and poor glycemic control. There are currently a paucity of data on the role of gender on coagulation activation and glycemic control in patients with type 2 diabetes mellitus in Enugu Metropolis. The present study was therefore designed to compare some markers of coagulation activation and glycemic control in patients with type 2 diabetes mellitus based on differences in gender.
Study setting
This study was conducted at the Enugu State University of Science and Technology Teaching Hospital Parklane, Enugu State, Nigeria. The teaching hospital is a major tertiary health facility located at the centre of the State metropolis (Enugu) for easy accessibility to residents. Enugu State derived its name from its capital and largest city, Enugu. It has an area of 7,161km2 with a population of 3,267,837 comprising mainly the Igbo tribe of the South Eastern Nigeria. It lies between longitudes 6o 30’E and 6o 55’E and latitude 5o 15’N and 7o 15oN. It consists of three senatorial divisions namely Enugu East, Enugu North and Enugu West and Seventeen Local Government Areas comprising 450 communities.
Study design
This was a prospective analytical cross-sectional study conducted on patients with type 2 diabetes mellitus (T2DM) with ages between 40-80 years in the Diabetic Outpatient Clinic from January 2022 to February, 2023 using the convenient sampling technique. A total of 100 T2DM patients were recruited for the study.
Ethical consideration
The ethical clearance to conduct this study was obtained from the Ethics Committee of the Enugu State University of Science and Technology Teaching Hospital (ESUTH), Enugu, with reference number. Informed consent was obtained from all participants before being recruited for the study.
Sample size
The sample size was calculated using the cross-sectional sample formula as applicable to G-Power software version 9 (G Power, Dusseldorf, Germany) Power analysis for one-way analysis of variance was conducted to determine a sufficient sample size using an alpha of 0.05, a power of 0.95 and a large effect size (f = 0.40). Based on this, the calculated minimum sample size of 44 subjects gave a 95% power to detect a difference of 0.40 at a significance level of 0.05 based on 8.0% prevalence of T2DM in the South Eastern Nigeria and 58% prevalence of coagulation abnormality in T2DM patients13,14.
Inclusion criteria
• Only T2DM patients were recruited.
• Patients between the ages of 40-80 years were recruited.
• Both male and female patients were recruited.
• Patients with BMI (kg/m2) between 18.5 – 28.5 were recruited.
Exclusion criteria
• Those with history of blood coagulation disorders were excluded.
• Patients with current history of anticoagulant therapy and oral contraceptive pills were excluded.
• Pregnant and lactating mothers were excluded.
• Patients with signs and symptoms of any pathological condition such as thromboembolic events, liver disease, renal disease, psychiatric illness and malignancies were excluded.
• Smokers, prolonged immobilized patients and paralysed patients were excluded.
Data and sample collection
The medical history and clinical data of the subjects were obtained using administered questionnaires and information from subject’s folder. Ten milliliters (10ml) of venous blood samples were collected from subjects following standard venipuncture technique. About 1.8ml was dispensed into a trisodium citrate container. The plasma was separated by centrifugation of the samples at 5000 revolution per minute for 15 minutes for the determination of the PT, INR and APTT while the remaining was dispensed into plain sample bottles and allowed to clot at room temperature; the serum was separated by centrifugation of the samples at 5000 revolution per minute for 5 minutes and stored at about -20oC for the determination of the fibrinogen, von willebrand factor and D-dimer levels. Subjects were also advised to observe overnight fast prior to collection of 2mls of blood for the estimation of FBS as well as another 2mls of blood immediately after 2 hours of eating for the estimation of 2HPPS while another random 2mls was collected for the estimation of HBAIC.
Determination of the activated partial thromboplastin time, prothrombin time and INR
The Activated Partial Thromboplastin Time and the Prothrombin Time were determined using the Manual method with plasma scann reagent obtained from Fortress Diagnostic Ltd, UK.
The INR was calculated using the formula
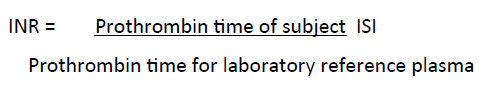
Where ISI stands for International Sensitivity Index for the plasmascann reagent.
Determination of the VWF, fibrinogen and D-dimer
The VWF, fibrinogen and D-dimer levels were determined using the Sandwhich Enzyme Linked Immunosorbent Assay Protocol with kit obtained from Shanghai Huirui Chemical Technology Co. Ltd, China and the Microplate Reader, Mindray-96A, China.
Determination of glucose
Glucose oxidase method was used to estimate the level of fasting plasma glucose and 2 hours post prandial plasma glucose with commercially available kit (Biologo, France) which has the standard for calibration measured at 500nm using spectrophotometer (SM 23A, England).
Determination of glycated hemoglobin
This was performed using the Ion Exchange Chromatographic Method with reagents obtained from Fortress Diagnostic Kits, England with values measured at 415mm using spectrophotometer (SMA 23A).
Statistical analysis
Statistical analyses was performed using the statistical package for social sciences software SPSS for windows, version 22.0 Armonk, NY, USA). The variables were investigated using Kolmogorov-Smirnov Test to determine whether they were normally distributed. Data was presented using means and standard deviations from the means. Oneway analysis of variance was applied to compare the markers of glycemic control and coagulation activation among the blood groups while the Student Test was used to compare between groups.
(Table 1) shows the comparison between the mean values of the markers of glycemic control for the subjects. There was no significant differences in the fasting blood glucose and two hour post prandial glucose (153±5.21, 247±20.63 respectively) for the female subjects compared to the male subjects (152±5.29 and 248±21.40) (p=0.569 and 0.228 respectively).The glycated hemoglobin was significantly increased (11.33±2.20) in the female subjects compared to males (p=0.031).
| Parameters | Females (n=50) |
Males (n=50) |
T-test (p-value) |
|---|---|---|---|
| FBS (mg/dl) | 153± 5.21 | 152±5.29 | 0.569 |
| 2HPPS (mg/dl) | 247 ± 20.63 | 248±21.40 | 0.228 |
| HBAIC (%) | 11.33 ± 2.20 | 7.21±2.26 | 0.031* |
| Age (years) | 60.30± 2.19 | 61.00±2.3 | 0.61 |
| Duration (years) | 3.12±1.93 | 3.40±2.01 | 0.133 |
| BMI (kg/m2 ) | 25.90±4.4 | 26.10±4.25 | 0.229 |
(Table 2) shows the comparison of the mean values of the markers of coagulation activation for the subjects. There was no significant differences in the prothrombin time, international normalized ratio, fibrinogen and D-dimer (10.24±5.60,0.37±3.10,8.60±2.23 and 280±54.9) for the female subjects compared to (10.08±4.40, 17.77±4.62, 7.69±2.19, 279±46.3) respectively for the male subjects (p=0.361, 0.626, 0.689, 0.418,0.173). There was significant difference in the activated partial thromboplastin time and von willebrand factor levels (32.46±3.41 and 28.27±5.43) for the females compared to the males (38.91±2.23 and 17.77±4.62) (p=0.003 and 0.002 respectively).
Parameters |
Controls (n=50) |
Cases (n=50) |
T-test (p-value) |
|---|---|---|---|
| APTT(secs) | 32.46 ± 3.41 | 38.91±2.23 | 0.003* |
| PT(secs) | 10.24 ± 5.60 | 10.08±4.40 | 0.626 |
| INR | 0.37 ± 3.10 | 0.39±2.20 | 0.689 |
| VWF(ng/ml) | 28.27 ± 5.43 | 17.77±4.62 | 0.002* |
| Fibrinogen(ng/ml) | 8.60±2.23 | 7.69±2.19 | 0.418 |
| D-dimer(mg/ml) | 280±54.9 | 279±46.3 | 0.173 |
| Age(years) | 60.30±2.19 | 61.00±2.3 | 0.61 |
| Duration(years) | 3.12±1.93 | 3.40±2.01 | 0.133 |
| BMI(kg/m2 ) | 25.90±4.4 | 26.10±4.25 | 0.229 |
There is no significant difference in the mean age of the subjects, duration of disease and the basal metabolic index for the female subjects (60.30±2.19,3.12±1.93,25.90±4.4) compared to the male subjects (61.00±2.3,3.40±2.01,26.10 ±4.25) (p=0.610,0.133 and 0.223).
Key: FBS = Fasting Blood Sugar, 2HPPS= Two Hours Post Prandial Sugar, HBAIC= Glycated Hemoglobin, BLR = BMI=Body Mass Index, *significant at p<0.05, Data expressed as Mean±SD.
Key: APTT = Activated Partial Thromboplastin Time, PT= Prothrombin Time, INR= International Normalised Ratio, VWF = Von Willebrand Factor, *significant at p<0.05, Data expressed as Mean±SD.
Sex hormones, gendered health norms as well as psychosocial based norms may have an influence on energy balance and glucose homeostasis resulting in differences in coagulation activation and glycemic control in male and female patients with T2DM (Misra R 2009).In the present study, we found significantly higher glycated hemoglobin levels (a marker of glycemic control) for the female subjects compared to the male subject. This is not in agreement with the findings of other studies who recorded no association between gender and glycemic control(Fox KM 2006) (Shah BR 2005). But is in agreement with other studies in literature which had reported poor glycemic control in women compared to men(Nilsson PM 2004) (Wexler DJ 2005). The suggested cause of poor glycemic control in women compared with men includes differences in regulation of homeostasis, treatment response and psychosocial factors(Duarte FG 2019).
We also found significant differences in the markers of coagulation activation involving the activated partial thromboplastin time and von willebrand factor levels between the male and female subjects. This is not in agreement with the findings of some studies who had reported no significant differences in the markers of coagulation activation between male and female subjects (Dayer MR 2014)( Zambia H 2019) But in agreement with some other studies who reported higher risk of hypercoagulability in female subjects compared to males (Odunsan O 2018) (Getu F 2018).The suggested gender based differences in the markers of coagulation activation in T2DM are also due to differences in homeostasis, treatment response and psychosocial factor (Marlic-Bilkan C 2017) (Rabei SS 2020).A limitation to the present findings is our small sample size and the cross sectional model for the study. We therefore recommend a large prospective study to substantiate the present findings.
The findings of the present study shows that gender has a great impact on coagulation activation and glycemic control in type 2 diabetes mellitus with females being more susceptible to poor glycemic control and coagulation activation compared to males.This findings suggests the need to develop a gender based management guideline for patients with type diabetes mellitus.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref