

Research Article - International Research Journal of Engineering Science, Technology and Innovation ( 2025) Volume 10, Issue 1
Received: 07-Feb-2024, Manuscript No. irjesti-24-127194; Editor assigned: 09-Feb-2024, Pre QC No. irjesti-24-127194 (PQ); Reviewed: 23-Feb-2024, QC No. irjesti-24-127194; Revised: 08-Apr-2025, Manuscript No. irjesti-24-127194 (R); Published: 15-Apr-2025, DOI: 10.14303/2315-5663.2025.116
The vegetable oils are one of the most perspective renewable raw materials due to bio- degradability and non-toxicity inherent them and also wide area of application. The non-edible seed oil of the Jatropha plant is a renewable and sustainable material to produce vegetable oil-based epoxy as raw polymeric material. The objective of this study is to synthesis Jatropha seed oil-based epoxy resins through conventional methods. An epoxy ring of Epoxidized Jatropha Oil (EJO) was formed through an epoxidation process using hydrogen peroxide and formic acid as an oxygen donor and oxygen carrier respectively. The physical characteristics of seed and epoxidized oil were investigated and also the oil was investigated by using analytical methods. The proximate compositions of the seeds were as follows: Moisture (4.128%), ash (3.4%) and seed density (1.4 g/ml). In addition to this, physicochemical characteristics of Jatropha seed oil were acid value (3.847 mg KOH/g, free fatty acid (1.9235 mg KOH/g). The jatropha seed oil can be suitable for the production because this give semidrying property. The epoxy oil was produced by mixing the jatropha oil, formic acid, hydrogen peroxide and sulfuric acid as a catalyst in three necked and stirred by magnetic stirrer in heating mantle for five hours. Then the product purified by washing with water.
The experiments were carried out by three-level and two-factor. A total of 9 experiments were conducted at conditions of reaction temperature 40, 55 and 70°C, volume ratio of hydrogen per oxide to oil 1, 1.5 and 2 and by taking the formic acid and sulfuric acid constant for 5 hours of reaction time.
Jatropha, Epoxidation, Epoxidized jatropha seed oil, Formic acid, Hydrogen peroxide
Epoxy resins were first commercialized in 1946 and are widely used in industry as protective coatings and for structural applications, such as laminates and composites, tooling, molding, casting, bonding and adhesives and others (Ahmad, et al., 2016).
Petroleum is the major source for production of polymers, plasticizers, lubricants and others (Biji, et al., 2010). The depletion of impacts. Bio resources derived from plant oil are an excellent substitute because they are available in abundance and green (Parthiban, et al., 2011).
Vegetable oils can be chemically modified to a value petroleum supply and the growing demand for new feedstock in many countries have encouraged researchers to find alternative resources. Plant oils and their derivatives have attracted the attention of researchers in various fields due to their availability, non-toxicity and, most importantly, modifiability by chemical, physical, or enzymatic methods (Saurabh, et al., 2011). Moreover, the usage of synthetic epoxy resin derived from petroleum raises many safety issues with regards to health and environmental added product by a complicated reaction called ‘epoxidation’. Due to the high reactivity of the oxirane ring epoxides can also act as a raw material for synthesis of variety of chemicals such as alcohols (polyols), glycols, olefin compounds, lubricants, plasticizer and stabilizer for polymers and their demand is increasing day by day (Sayyar, et al., 2009). Vegetable oil represents one of the cheapest and most abundant biological feedstock available in large quantities and its use as starting material offers numerous advantages such as low toxicity and inherent biodegradability (Derahman, et al., 2019).
Jatropha grows in various parts of Ethiopia, as a hedge around homesteads and farmlands, such as in Wolayita, Metekel, Southern Wollo, Northern and Eastern Shoa, Tigrai, Gamo Gofa zones and Gambella region (Yadav, et al., 2020). This suggests that Jatropha can be cultivated either as large-scale plantations on marginal areas, as small-scale hedges, or intercropped to assist rural livelihoods (Khalil, et al., 2013). Although Jatropha already exists in many places of Ethiopia; its economic importance is far from being realized due to absence of proper evaluation and promotion of the existing local Jatropha provenances for their oil content, oil yield and oil quality for biodiesel utilization.
The excellent chemical properties, performance characteristics and ignition quality equivalent to diesel makes Jatropha oil a promising and sustainable alternative for diesel to overcome the source limitation problem [1-3].
Sample collection and preparation
The required raw material (1.5 kg), jatropha seed, was collected from Gofa Sawla and Metekel. It was cleaned by removing the unnecessary materials and washed to remove the soil. Then it dry in oven at 105°C for 5 hr. The seeds were further dried in order to reduce its moisture content, it dried up to 3%-5%. The dried and cleaned jatropha seed was grinded by using simple grinding machine.
Moisture content of the seed
The empty dish was weighed with and without the amount of seed and dried in an oven at 105°C for 5 hr, weighing each until constant weight is obtained and finally the weight was taken and compared with the initially recorded weight. The percentage weight in the seed was calculated using the formula.
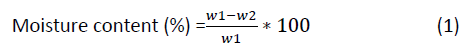
Where;
W1=Original weight of the sample before drying;
W2=Weight of the sample after drying.
Ash content of the seed
The empty crucible was weighed with and without the amount of dried seed and put in to Furnas at 600°C for 1:30 hr finally the weight was taken and compared with the initially recorded weight. The percentage weight in the seed was calculated using the formula.
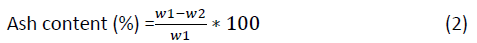
Where;
W1=Original weight of the sample before burned
W2=Weight of the sample after burned
Determination of effect of size reduction
The moisture was removed by placing the sample in an oven at 105°C for 5 hours. The dried jatropha seed was crushed manually by hand and pestle to separate the seed from the flake. Then dried and cleaned jatropha seed was grinded by using simple grinding machine.
The sample was sieved using with set of sieves sizes arranged in descending order 120 mesh 60 mesh and 35 mesh to obtain particular sizes of jatropha seed powder. This was tried to investigate the effect of particles size on yield and quantity of oil.
Extraction of jatropha oil
20 gram of the powder jatropha was placed in the soxhlet and 200 ml of hexane were poured into the round bottom flask, the apparatus was heated at 68°C and allowed for 5 hrs. Then, after 5 hours, the required mixture of jatropha oil and Hexane was obtained. The mixtures of jatropha oil and hexane were separated by using rotary evaporator for 30 min. Then the required jatropha oil was obtained (Figure 1) [4,5].
Figure 1. Soxhlet extractor, rotary evaporator and Jatropha oil.
Characterization of the jatropha oil
Acid value of jatropha oil: The number of mg of KOH required to neutralize the free acids in 1 g of the oil will be determined by placing 0.5 g of sample in conical flask containing mixture of ether and ethanol (50 mL; 95% v/v). Standard alcoholic potassium hydroxide solution (0.1 N) was prepared by dissolving KOH with distilled water.

Where: V=ml of 0.25 M KOH consumed by sample
N=concentration of KOH
W=weight in grams of the sample (jatropha oil)
56.1=molecular mass of KOH
pH of jatropha oil
The pH meter was inserted into the jatropha seed oil and the pH reading was recorded [6-8].
Density of the jatropha oil
50 ml of the oil was taken. The weight of the beaker used to hold the oil was measured and add the oil in to it. Then the total mass of the beaker and the oil was measured and recorded. Then the mass of the beaker was subtracted from the total mass of the oil and the beaker. Now, the mass of the oil was obtained. After we know the mass of the oil we can determine the density of the oil by using density formula as follows.

Specific gravity of the jatropha oil
The density of the oil was determined by measuring the weights of oil produced in laboratory divided by the volume of the oil. Then to determine specific gravity of oil by dividing density of oil by the density of water.

Percentage free fatty acid in the jatropha oil
Percentage Free Fatty Acids (FFAs) were calculated as a factor of acid value as FFAS=(28.2*V*N)/W
%FFA*1.99=Acid value
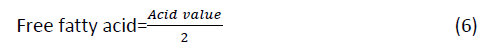
Percentage oil yield of jatropha
Is the ratio of mass of the oil to the mass of the sample?
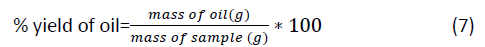
Epoxy production procedure
The epoxidation method was takes placed as follows. 10 ml JO was placed in them three necked glass flask. 15 ml of formic acid and 1 ml of sulphuric acid were added. The mixture of oil, formic acid and sulphuric acid was stirred by magnetic stirrer for 30 min in heating mantle at a temperature of 55°C. Then the requisite amount of 15 ml aqueous hydrogen peroxide was added drop by drop, at such a rate that the addition was completed in half an hour. After complete addition of hydrogen peroxide, the reaction was continued further for 5 hours’ time duration. Utmost care was exercised during operation so as to keep the reaction mixture under stirring; this was required for avoiding zones of high concentration of peroxide that could lead. The course of reaction was followed by withdrawing aliquots, the first being taken after an hour, the addition of hydrogen peroxide being considered zero time. Prior to analysis, the samples were extracted with diethyl ether, washed with water until they were acid-free and analyzed for oxirane content and iodine value.
Characterization of physical property of epoxy
The quality and the quantity of epoxy oil was determined based on its property. The physical and chemical properties of epoxidized jatropha oil was determined during analysis time.
Density of epoxy
20 ml (20.54 g) of the epoxidized oil was taken. The weight of the beaker used to hold the oil was measured and add the oil in to it. Then the total mass of the beaker and the oil was measured and recorded. Then the mass of the beaker was subtracted from the total mass of the oil and the beaker. Now, the mass of the oil was obtained. After we know the mass of the oil we can determine the density of the oil by using density formula as follows.

pH of the epoxy
The pH meter was inserted into the jatropha seed oil and the pH reading was recorded.
Odor
Smell is created when the resin begins to harden. The fumes that are released will be stronger when the epoxy resin is first mixed and will decrease as the mixture begins to harden.
Colour
This described by looking the product. Most of the time epoxy are colorless.
Specific gravity
The density of the epoxidized oil was determined by measuring the weights of oil produced in laboratory divided by the volume of the oil. Then to determine specific gravity of oil by dividing density of epoxidized oil by the density of water.

Proximate analysis of jatropha seed
The physical parameters of the seed affect chemicals that are contained either in pulp or kernel that directly. The moisture content is the amount of water in the seed and is usually expressed as a percentage. It can be expressed on either a wet weight basis or on a dry weight basis. The moisture content of the jatropha seed was determined on dry basis. The measured ash and the moisture content of the seed respectively are comparable with the values that reported by Biji. Which are 4.128% and 3.4% respectively. But the density of jatropha seed which determined had little difference with the standard value. This may be due to the nature of seed, variety or the environment in which the seed grow as well the types of seed (Table 1) [9,10].
| Characteristics | Result | Standard value |
| Moisture content | 4.13% | 3%-5% |
| Ash content | 3.40% | 3.20% |
| Density | 1.4 g/ml | 0.926 g/ml |
Table 1. Proximate analysis of jatropha seed.
Physicochemical analysis of jatropha oil
The acid value of jatropha seed decreases with increasing the particle size of the seed this indicates that the oil which extracted from finer particle size is more acidic. As we know theoretically as the impurity of the oil increase the acidity of the oil also increase so this result show that the oil from finer particle size is more impure (Table 2).
Characteristics |
Result |
Standard value |
Density |
0.8932 g/ml |
0.920 g/ml |
PH |
5.53 |
5.2 |
Specific gravity |
0.893 |
0.91 |
Acid value |
3.847 mg |
1.16 mg/KOH-1 oil |
Percentage of oil yield |
29.2 |
39-46 |
Percentage of free fatty acid |
1.9235 |
0.58 |
Table 2. Characterization of jatropha oil.
The measured value of specific gravity and density that indicate the physical characteristics of the oil respectively are 0.8932 and 0.91 which is clothes to the standard value 0.91 and 0.920 g/ml that is compared with the standard such value variation across species and locations might be attributed to the environment and geological conditions of varied regions. The pH is the degree of the acidity of the oil. The pH value for jatropha oil was found to be 5.58 and is near to the value reported by Biji. An acid value is indication of the age and quality of the oil or fat. The acid value was determined to be 3.8 mg KOH/g which implies low fatty acid content. The result obtained is within standard. The percentage free fatty acid value indicates the deteriorating condition and edibility of the oil. Low value (1.9235%) is obtained for the free fatty acid as indicated in table 3 and is in the range that reported by and stearic acid (2.32.8%).
Physical characterization of epoxidized jatropha oil
Density, PH, odor, color, density, specific gravity of the epoxy was measure and the results are listed below in the table (Table 3).
| Characteristics | Result | Standard value |
| Density | 0.978 g/ml | 1.1 g/ml |
| PH | 1.94 | 2.24 |
| Color | Milky white | Colorless |
| Odor | Unpleasant | Unpleasant |
| Specific gravity | 0.978 | 1.5 |
Table 3. Physical characteristics of epoxy.
In characterizing the physical property of the epoxidized jatropha oil which is PH, density, color, odor and specific density were comparable with the standard.
But it has a little bit different due to limitations of the production process like the effect of temperature, stirrer and material used in the process and also when the washing or recovering of sulfuric acid doesn’t completely do.
In addition to this to underestimate the quality of the product we need to know the oxirane oxygen content, iodine value and other chemical properties of the product but due to the limitation of chemicals used to characterize we didn’t check the result so we only considered the physical properties of the product and also our result was comparable with the standard value.
Effect of hydrogen peroxide and temperature of jatropha oil
The effect of temperature and hydrogen peroxide increases the adhesive property of the epoxy and also increases with an effective stirrer. When it considers our experimental result got a good result of the product at the temperature of 55°C from 40°C by taking the same volume ratio of hydrogen peroxide to oil ratio, formic acid and sulfuric acid. As listed in Dinda. Production of epoxy increase with in the temperature and hydrogen peroxide. The following graphs show the effect of H2O2/oil with density and temperature variation with density (Figure 2).
Figure 2. Effect of temperature and hydrogen peroxide.
In this research Jatropha oil was used as a raw material to produce epoxy. The production process was held from the collection of raw material (Jatropha seed) from Gammo Goffam and Metekel, Ethiopia and the collected seed was prepared to extract oil from it, after preparation the seed was characterized. Then oil was extracted by using solvent extraction (N-hexane). To extract the oil (0.125-0.25, 0.25-0.5 and 0.5-1.68) particle size are used as factor to analyse the yield and quality of oil. Followed by extraction of oil the physic chemical characteristics was determined like density, specific gravity, acid value and free fatty acid value. Finally, epoxidized jatropha oil was produced in the production process, sulfuric acid, formic acid and hydrogen peroxide was used as a catalyst. Oxygen carrier and oxygen donor respectively and nine experiments are done by taking volume ratio of hydrogen peroxide to oil and reaction temperature as a factor with three level (1:1, 1.5:1 and 2:1 for H2O2 to oil ratio and 40°C, 55°C and 70°C for reaction temperature) and then the final product of epoxidized jatropha oil was characterized like density, PH, odor, color and adhesive property.