

Research Article - International Research Journal of Plant Science ( 2021) Volume 12, Issue 2
Received: 02-Apr-2021 Published: 26-Apr-2021, DOI: 10.14303/irjps.2021.008
The present study was done from the month of January to June in 2019. The aim of this study was to investigate the impact of Domestic wastewater (DWW) on the growth of Phragmites karka and Chrysopogon zizanioides in Constructed wetland and phytoaccumulation of heavy metals. The fresh weight, dry weight, moisture content, relative growth rate and absolute growth rate of plants were calculated by Classical and functional methods. The concentrations of metals were determined by AAS. The wastewater contained higher concentration of Fe and Zn in comparison to Cu and Pb and calculated concentration of Heavy metals in DWW ranged from 2.45 ± 0.01 to 2.92 ± 0.05 for Fe; 70.73 ± 1.2 µgL-1 to 88.33 ± 0.58 µgL-1 for Zn; 0.62±0.01 µgL-1 to 1.09±0.01µgL-1 for Pb and 1.83±0.03 µgL-1 to 2.6±0.12 µgL-1 for Cu. C. zizanioides produced huge amount of biomass in comparison to P. karka. Both plants played an important role in scavenging the metals from the wastewater. The accumulations of metals increased with the increases in dry mass. From that experiment, it could be suggested that application of both the plants in constructed wetlands for accumulation of heavy metals is a viable option for reclamation of toxicity of metals.
Domestic wastewater, growth, heavy metals, P. karka, C. zizanioides.
The development of industries, mining activities, irrigation of croplands with wastewater, and the application of sewage sludge into land increased the release of metals into the earth’s ecosystems (Dotaniya, et al., 2018). Heavy metals in the soil and water are considered as powerful tracers for monitoring the impacts on human activities (He et al., 2005) that poses a threat to human population, fauna and flora (Nagajyoti, et. al 2010). Wastewater irrigation acts as a significant contributor of heavy metal contents to soil (Mudhiriza et al., 2015). Replacement of essential nutrients by heavy metals at cation exchange sites of plants is indirect toxic effect of metals (Taiz and Zeiger, 2002). Heavy Metals at their toxic levels within a plant obstruct their normal functioning and acts as an obstacle to various metabolic processes in a variety of ways. It causes the disturbance or displacement of protein structure (Hall, 2002) and hinders the formation of functional cellular molecules (Hussain et al., 2019). These metals also supersede the functions of essential metals in biomolecules like pigments or enzymes.
India, like other developing countries, requires economical and cost-effective alternatives for wastewater treatment. The degree of growth is affected by canopy structure which includes its height, distribution of leaves and storage and accumulation of material (Ordog & Molnar, 2011). The analysis of Plant growth is a quantitative method which is used to interpret the performance of plants growing under natural and controlled conditions. The plant growth is accompanied by a quantitatively change in biomass (Amanullah, et al. 2008). Change in sowing time brings out notable variations in plant growth.
The growths of both the plants were computed by the calculation of total fresh weight of whole plant, dry weight of whole plant organs and increased length of whole plants. Emergent plants are used as the natural catalysts to adsorb, absorb and accumulate heavy metals in their tissues from polluted water and soil (Vymazal and Kropfelova, 2008). The macrophytes with strong absorption for pollutants and good tolerance could be planted in constructed wetlands which accordingly removes or fix water pollutants through adsorption, absorption, accumulation and degradation (Wang et al., 2012). They provide good conditions for physical filtration and large surface area for attached microbial growth and activity (Brix, 1997). The basic component in the wastewater treatment in constructed wetlands is the integrated combination of physical, chemical and biological interactions among biotic and abiotic components of the ecosystem. (Kyamabadde et al., 2005).
As water pollution is growing continuously and various measures have been taken to overcome the problem for which various technologies have been employed. The removing of pollutants through the application of plants had gained importance and various plants have been proved to be efficient in waste water management. On seeing the potential of Chrysopogon zizonioides and P. karka in phytoremediation and removal of pollutants from different wastewater sources, the present study was done to evaluate the impact of Domestic wastewater on growth of Chrysopogon zizanioides and Phragmites karka in Constructed wetland and their heavy metals extraction ability. There had been a lot of changes in physico-chemical characteristics of domestic wastewater and performance efficiency of both plants had been evaluated. Treated water can be utilize for industrial processes, household activities, irrigation purposes like agriculture, horticulture, gardening, social forestry.
Gwalior is located at 26.220 North 78.180 East in northern M.P one among four Raj Bhogi cities of Moha Nagaras of Madhya Pradesh (Koul et al., 2012).
Site Selection
The present work on phytoextraction of heavy metals from domestic wastewater and growth parameters of Phragmites karka, and Chrysopogon zinzanoides was carried out at School of Studies in Botany, Jiwaji University Gwalior.
Starting of Experiment
Both plants i.e. Phragmites karka and Chrysopogon zizanioides were planted in wetlands beds during the 1st week of January 2019. Well rooted slips of both plants were selected and placed 5cm inside the substratum. The uniform gaps were maintained between the slips. Growths of the plants were monitored till the last week of June. The wastewater from open drainage system of Mahalgaon Gwalior was allowed to move into a settling tank of 750 liters of capacity. The effluent of settling tank was used as influent for 3 treatment beds. Three treatments set up beds were as follows: • Constructed wetland without any plant (CWWP). • Constructed wetland planted with Phragmites karka (CWPK) • Constructed wetland planted with Chrysopoigon zizanioides (CWCZ).
Heavy Metals Analysis
Samples of wastewater were collected into distilled sterile 2-L polylab bottles and immediately transported to the laboratory. Heavy metals of samples were analysed by Atomic Absorption Spectroscopy method (Matejovic & Durackova, 1994; Hseu, 2004). The water samples taken for heavy metal analysis were filtered and poured into an evaporating dish followed by acidification with 1 mL HNO3 . The samples were boiled to expel oxides and chlorine and analysis of heavy metals were done by using Atomic Absorption Spectroscopy (Perkin Elmer AAnalyst 700 model) AAS. The readings that displayed on instrument were noted down respectively.
Plant Growth Parameters
The growth of plants was calculated by “Classical Approach”. It is one of the oldest methods that were introduced by (Blackman, 1919; West et al., 1920). The heights of plant samples were measured by Classical methods (Heady, 1957; Reich, 2000) using meter rule at monthly intervals. The total fresh weight and dry weight of plants were calculated by Classical method (Asaduzzaman et al., 2010). The moisture content of Plant samples were estimated by Drying Oven method (American Society for Testing and Materials) D4442 (Reeb and Milota, 1999). Absolute Growth Rate (AGR) was calculated by classical and functional methods as modified by Causton and Venus (1971) (Hunt, 1990). It gives Absolute values of biomass between two time intervals.
Calculation
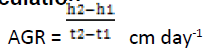
Where
h1 and h2 are the plant height
t1 and t2 are different time periods respectively.
Relative Growth Rate (RGR) was coined by Williams (1946) and it was calculated by classical and functional methods as modified by Causton and Venus (1971) (Hunt, 1990). The measurement is based on the dry mass for whole plant that also includes roots. It is expressed as unit dry weight/unit time (gg-1day-1). Calculated by below given formula:
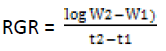
Where,
W2 and W1 are whole plant dry weight at t2 and t1
respectively
t2 and t1 are time interval in days.
Plant tissue digestion and Heavy Metal analysis
2 grams of dried plants sample were digested by destruction and dry ashing method (Lenssen et al. 1999). The pre-weighed plant samples were taken into porcelain crucibles and subsequently ashed at 300 0 C for 3 hours in muffle furnace. The residue of plant samples were dissolved in 5 ml of 3 M HNO3 (AR grade). Finally, the digested samples were filtered through Whatman No. 41 filter paper into a 50 ml volumetric flask. Subsequently distilled water was added to it to make the final volume as 50ml. Metal analyses were conducted via an atomic absorption spectrophotometer (AAS, Perkin Elmer). The reading were recorded which displayed on instrument.
Calculation
Metal (ppm or ppt) = (ppm in extract – blank) x 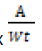
Where,
A = Total volume of extract (ml)
Wt = Weight of dry plant (g)
The Domestic Wastewater carries an appreciable amount of toxic heavy metals. The concentration of heavy metals varies from city to city. They are non-biodegradable substances and could readily accumulate both in soil and organisms upto toxic levels. So long term application of heavy metals on land in any form results in the elevated levels of heavy metals in soil (TytÃ…?a, 2019). The concentration of DWW ranged from 2.45±0.01 mgL-1 to 2.92±0.05 mgL-1 for Fe; 70.73±1.2 µgL-1 to 88.33±0.58 µgL-1 for Zn; 0.62±0.01 µgL-1 to 1.09±0.01µgL-1 for Pb and 1.83±0.03 µgL-1 (Jan) to 2.6±0.12 µgL-1 for Cu while concentration of metals in Settling effluents varied from 2.14±0.01 mgL-1 to 2.45±0.03 mgL-1 for Fe; 5.52±1.6 µgL-1 to 18.75± 1.2 µgL-1 for Zn; 0.49±0.02 µgL-1 to 0.8±0.02 µgL-1 for Pb and 1.6±0.06 µgL-1 to 1.97± 0.09 µgL1 for Cu (Table 1).
| Sites | Parameters | Jan | Feb | Mar | Apr | May | Jun | |
|---|---|---|---|---|---|---|---|---|
| DWW | Fe (mgL-1) | 2018 | 2.89±0.04 | 3.18±0.01 | 3.18±0.01 | 2.54±0.04 | 2.56±0.01 | 2.89±0.01 |
| 2019 | 2.51±0.04 | 2.92±0.05 | 2.92±0.05 | 2.71±0.04 | 2.45±0.01 | 2.64±0.05 | ||
| Zn (µgL-1) | 2018 | 86.67±1.86 | 77.87±2.14 | 84.33±5.36 | 81.37±3.86 | 87.77±2.39 | 72.67±1.85 | |
| 2019 | 76.67±1.86 | 88.33±0.58 | 87±2.08 | 81.50±1.46 | 70.73±1.20 | 72±2.2 | ||
| Pb (µgL-1) | 2018 | 1.07±0.03 | 0.77±0.01 | 0.58±0.01 | 0.65±0.02 | 0.61±0.02 | 0.72±0.01 | |
| 2019 | 1.09±0.01 | 0.99±0.01 | 0.75±0.02 | 0.73±0.01 | 0.62±0.01 | 0.70±0.01 | ||
| Cu (µgL-1) | 2018 | 2.00±0.12 | 2.23±0.2 | 2.77±0.23 | 2.23±0.09 | 2.57±0.09 | 2.27±0.09 | |
| 2019 | 1.83±0.03 | 2.13±0.07 | 2.60±0.12 | 2.13±0.12 | 2.20±0.17 | 2.40±0.06 | ||
| ST | Fe (mgL-1) | 2018 | 2.56±0.02 | 2.89±0.04 | 2.89±0.04 | 2.15±0.01 | 2.3±0.01 | 2.58±0.01 |
| 2019 | 2.42±0.06 | 2.40±0.11 | 2.40±0.11 | 2.24±0.01 | 2.14±0.01 | 2.45±0.03 | ||
| Zn (µgL-1) | 2018 | 74.70±0.85 | 73.07±0.91 | 72.96±1.96 | 66.3±2.97 | 73.22±1.04 | 61.40±0.58 | |
| 2019 | 72.37±0.55 | 79.40±0.59 | 73.93±0.91 | 66.21±1.48 | 69.37±0.90 | 61.35±1.20 | ||
| Pb (µgL-1) | 2018 | 0.87±0.00 | 0.60±0.01 | 0.46±0.01 | 0.59±0.01 | 0.49±0.01 | 0.43±0.01 | |
| 2019 | 0.75±0.03 | 0.80±0.02 | 0.64±0.01 | 0.65±0.02 | 0.51±0.01 | 0.49±0.02 | ||
| Cu ( µgL-1) | 2018 | 1.73±0.03 | 1.9±0.12 | 2.03±0.12 | 1.77±0.09 | 1.73±0.09 | 1.93±0.03 | |
| 2019 | 1.6±0.06 | 1.82±0.04 | 2.10±0.05 | 1.77±0.03 | 1.7±0.06 | 1.97±0.09 |
Turek et al. 2019 reported the concentration of Cu (126.9- 143.5 mgKg-1), Pb (27.6-35.7 mgKg-1) and Zn (843.1-986.5mgKg-1) in Sewage Sludge which is totally different as compared to the results observed. Dheri, et al. (2007) reported higher concentrations of Pb in sewage wastewater while concentration of Pb was similar in the water drawn from deep tube well. The calculated results of Fe, Zn, Cu and Pb depicted that the DWW before treatment is suitable for irrigation according to FAO 1985/WHO 2005/APHA 2002 (Ayers & Westcot, 1994; Amoah et al., 2005).
Plant Growth Parameters
Both plants P. karka and C. zazanioides produced huge amount of biomass in experimental setups like other grasses. The plants grow strongly as tufted grass with an extensive branching rhizome and stout culms. There was a continuous branching of rhizome and increment in numbers of culms per plant in reed grass while huge amount of dense clumped fibrous root system with stout erect solid glabrous culms developed in vetiver grass.
The number of culms ranged from 12 to 16 per rhizome in P. karka till June whereas 24 to 32 culms per plant were developed in C. zizanioides. Lot of change was found in plant height right from plantation till last sampling. The change in size of P. karka ranged from 9.33±0.88cms in Jan 2019 to 184.33±5.81cms in Jun 2019 (Table 2 & Figures 1-5) with highest AGR in the month of February i.e. 1.81±0.08cm day-1 (Table 3 & Figure 6). Similarly the length of C. zizanioides sapling at the time of cultivation was 19.4±1.81 which increased to 187.67±1.67 cms in June (Table 2 & Figure 5) with highest AGR in the February as 1.53±0.12 cmday-1 (Table 4 & Figure 7).
| Jan | Feb | Mar | Apr | May | Jun | ||
| Moisture content (%age) | P.K | 77.37±1.46 | 74.29±1.00 | 73.02±0.34 | 73.29±0.84 | 72.16±0.45 | 67.02±1.32 |
| C.Z | 78.32±0.51 | 73.69±0.42 | 73.32±1.30 | 73.26±0.27 | 71.71±0.44 | 64.37±0.61 | |
| Plant height (cms) | P.K | 9.33±0.88 | 22±2.65 | 76.33±5.32 | 115.33±4.67 | 147.33±3.28 | 184.33±5.81 |
| C.Z | 19.4±1.81 | 45.33±2.40 | 91.33±5.78 | 135±3.51 | 167.67±2.84 | 187.67±1.67 |
| Fresh weight (grams) | P.K | 2.41±0.12 | 6.14±0.27 | 12.18±0.58 | 29.71±1.22 | 38.58±1.13 | 49.33±1.45 |
| C.Z | 2.94±0.16 | 6.77±0.33 | 13.72±0.36 | 25.81±0.65 | 33.28±0.60 | 40.94±1.00 | |
| Dry weight (grams) | P.K | 0.54±0.10 | 1.58±0.11 | 3.29±0.16 | 7.93±0.39 | 10.75±0.46 | 16.22±0.15 |
| C.Z | 0.63±0.30 | 1.78±0.10 | 3.67±0.27 | 6.90±0.19 | 9.42±0.31 | 14.6±0.60 |
| Relative Growth Rate (RGR) in mmg- 1day-1 |
P.K | 15.26±0.35 | 10.63±0.47 | 12.76±0.81 | 4.40±0.12 | 6.01±0.68 |
| C.Z | 14.81±1.20 | 10.48±0.55 | 9.22±1.37 | 4.41±0.38 | 6.42±0.86 | |
| Absolute Growth Rate (AGR) in cmday-1 | P.K | 15.26±0.35 | 10.63±0.47 | 12.76±0.81 | 4.40±0.12 | 6.01±0.68 |
| C.Z | 14.81±1.20 | 10.48±0.55 | 9.22±1.37 | 4.41±0.38 | 6.42±0.86 |
The belowground and above ground density of C. zizanioides was found to be better than P. karka. The root system of C. zizanioides was comparatively deeper than P. karka. The plants steady increase in heights during first 3 months. Similar observations were reported Maurya et al (2016) while studying the plant height of Pennisetum glaucum L in Allahabad and similarly in T. latifolia (Golda-Arpudhalin, 2017). Weights of both the plants increased with time as reported from 1st to 6th month. The fresh weight of both the plants were measured once collected from the experimental setups. The calculated data showed the fresh weight per calm in P. karka from January to June varied from 2.41±0.12 grams to 49.33±1.45 grams (Table 4 & Figure 7). The fresh weight of P. karka was highly influenced when grown in 100mM NaCl ((Abideen et al., 2014). Similarly in C zizanioides, the change in fresh weight varied from 2.94±0.16 grams to 40.94±1.00 grams (Table 3 & Figure 7). Water content present in plants plays an important role during the process of photosynthesis, translocation of solutes and a number of other processes in plants.
The total dry weight of both the plant increased with time and the calculated results showed that change in dry weight for P. karka was 0.54±0.10 to 16.22±0.15 from January to June respectively (Table 3 & Figure 8) with highest RGR in the month of February as 15.26±0.35 mgg-1day-1 and least RGR in the month of May as 4.40±0.12 mgg-1day-1 (Table 4 & Figure 9). Similarly there was also great change in total dry matter of C. zizanioides as the variation in dry matter was found to be 0.63±0.30 in Jan 2019 to 14.6±0.60 in the month of Jun 2019 (Table 3 & Figure 8) with highest RGR in February as 14.81±1.20 mgg-1day-1 and least in month of May as 4.41±0.38 mgg-1day-1 (Table 4 & Figures 9,10). Similar fresh weight and dry weight increments were observed in A. donax and T. latofolia (Golda-Arpudhalin, 2017). During last two months, both the plants showed significant increase in dry weights as was observed by Maurya, et al., (2016) while they study the plant dry weights of various varieties of Pennisetum glaucum L in Allahabad. Almost similar results for RGR were shown by Brachiaria brizantha (palisade grass) when cultivated from Late autumn to early spring in Brazil (Giacomini et al., 2009). The RGR shown by Lathyrus sativus L (green pea) was 95mgg-1day-1 (Ghosh et al., 2018). The fast growing fern species Azolla microphylla and Azolla caroliniana showed 850mgg-1day-1 and 870mgg1 day-1 respectively.
There is a great impact of planting geometries, location and depths of planting of plants for AGR and relative growth rate (Rajput et al 2017). The AGR and RGR vary from genera to genera and from family to family as in case of Nigella sativa, the highest AGR was 0.0321 g day-1 and RGR was 0.0714gg-1day-1 (Roussis, et al., 2019). The P. vulgaris had shown 48.02gg-1day-1 RGR which is comparatively much higher than our calculated results. However, the plant had shown AGR as 0.1g day-1 which is less than our calculated results (Gebeyehu, 2019). P. karka and C. zizanioides displayed a classical time and dose dependent toxicity response to the contaminants present in DWW in growth which is similar to P. karka under different saline conditions (Shoukat et al., 2018) and other halophytic grasses (Ahmed et al., 2013).
The moisture content of a plant is calculated as the percentage of the dry weight to the fresh weight. Due to high concentration of salts in DWW, we observed a significant reduction in moisture content for all plants (Acosta-Motos et al., 2017). The calculated data showed that at the time of plant sapling plantation, the moisture content of P. karka culms were 77.37±1.46% which decreased to 67.02±1.32% in the month of June. Similarly the moisture content of C. zizanioides saplings at the time of plantation was 78.32±0.51% that it decreased to 64.37±0.61% in the month of June (Table 2 & Figure 6).
The observed results suggested that the fresh weight and dry weights were both increasing continuously. However, there was sudden decrease in moisture content and rapid increase in dry weight during last 2 months. Both the plant species displayed dose dependent salinity response in growth and regulated their biomass with higher relative water (Riccardi et al., 2014) which is already observed in other halophytic grasses (Ahmed et al., 2013). The moisture content of evergreen broad-leaved plant species were calculated as 51.3%±11.0% to 56.6%±8.5%. (Lifang et al., 2019). The moisture content of plants significantly varied at different growth stages.
Heavy Metals in Plants
Phytoremediation and accumulation of metals in plant body is considered as cost effective, aesthetically pleasing and environmental friendly. Plants accumulate metals contaminants all the way through their roots and translocate these metals in whole plant body (Ali et al., 2020). Absorption and subsequent accumulation of metals into plant tissues depends on temperature, moisture content, organic matter, pH of medium and availability of nutrients and other properties (Rupa et al., 2003). The accumulation of heavy metals per kilogram in plants increases with time upto saturation point.
The calculated results had shown the concentration of heavy metals in P. karka were increased from 30.82±0.65 mgKg-1 to 182.06±1.89 mgKg-1 for Fe; 2.60±0.01 mgKg-1 to 13.28±0.11 mgKg-1 for Zn; 0.122±0.009 mgKg-1 to 0.594±0.16 mgKg-1 for Cu and 0.022±0.002 to 0.078±0.003 for Pb. Similarly the concentration of metals in C. zizanioides increased from 34.95±1.31 mgKg-1 to 145.02±1.62 mgKg-1 for Fe; 2.83±0.16 mgKg-1 to 11.86±0.08±0.11 mgKg-1 for Zn; 0.11±0.003 mgKg1 to 0.52±0.009 mgKg-1 for Cu and 0.016±0.001 mgKg-1 to 0.058±0.001 mgKg-1 n 2019 for Pb (Table 5 & Figures 11- 13). The total dry biomass of both the plants was increased significantly in wetlands. The results revealed that both plants played an important role in scavenging the metals from the wastewater. The accumulations of metals increased with the increases in dry mass.
| Iron (mgKg-1) | PK | 30.82±065 | 87.55±0.25 | 122.34±0.32 | 135.65±0.23 | 163.85±4.01 | 182.06±1.89 |
| CZ | 34.95±1.31 | 68.01±0.43 | 76.52±0.71 | 117.23±0.71 | 143.98±1.76 | 145.02±1.62 | |
| Zinc (mgKg-1) | PK | 2.60±0.01 | 6.04±0.19 | 8.33±0.02 | 9.25±0.05 | 11.27±0.09 | 13.28±0.11 |
| CZ | 2.83±0.16 | 4.74±0.01 | 6.40±0.02 | 9.14±0.02 | 11.21±0.02 | 11.86±0.08 | |
| Lead (mgKg-1) | PK | 0.022±0.002 | 0.017±0.001 | 0.026±0.002 | 0.041±0.001 | 0.061±0.002 | 0.078±0.003 |
| CZ | 0.016±0.001 | 0.022±0.001 | 0.028±0.001 | 0.035±0.001 | 0.048±0.002 | 0.058±0.001 | |
| Copper (mgKg-1) | PK | 0.11±0.003 | 0.12±0.002 | 0.23±0.006 | 0.34±0.008 | 0.41±0.008 | 0.52±0.009 |
| CZ | 0.016±0.001 | 0.022±0.001 | 0.028±0.001 | 0.035±0.001 | 0.048±0.002 | 0.058±0.001 |
The highest concentrations of heavy metals in both the plants were observed during 6th month. During 1st to 4th the rate of accumulation was relatively very high in than last two months. Comparing the accumulation efficiency between the two plants, the P. karka showed better results for the accumulation of Fe while rest of the 3 metals were efficiently accumulated by C. zizanioides. Accumulation of heavy metals directly depends on the concentration of metals present in medium and requirement of elements in plant life processes (Singh et al., 2011).
The concentrations of heavy metals in DWW and plants accumulation followed the same order as: Fe > Zn > Cu > Pb. This reflects the bio-monitoring potentialities of both the plant species. Similar order in accumulation of heavy metals was observed by Oreochromis niloticus fish in Northern Delta Lakes, Egypt (Saeed, 2008) and carrot (Carota Sativus), radish (Raphanus Sativus) and spinach (Spinacea Oleracea) vegetables in Greater Noida India (Hussain et al., 2019). Similarly R. carnea accumulated 0.985±0.034 mgKg-1 Fe; 0.979±0.032 mgKg-1 Cu and 0.82±0.007 mgKg-1 Pb, from sewage water with average metals concentrations of water as 2.015 mgKg-1 for Fe; 2.301 mgKg-1 for Cu and 0.211mgKg-1 Pb (Zhang, et. al 2007). Both the plants accumulated higher concentrations of Fe and Zn and less concentration of Cu and Pb as compared to L. minor and L. gibba (Daud et al., 2018). Zinc is an essential trace element which plays an important role in the growth and development of plants. The plants showed efficient results for accumulation of Zn and Fe from DWW in comparison to T. domingensis. (Mojiri, 2012). E. pyramidalis and L. stolonifera accumulated comparatively higher concentration of Pb from water (Abd-Elaal et al., 2020) than P. karka and C. zizanioides.
The concentrations of metals accumulated by vegetable plants like lettuce, spinach, radish and carrot were less than our calculated results when these were cultivated in contaminated soil (Hussain et al., 2019). Alfaalfa (M. sativa) accumulated 1.9- 5.9mg/kg Cu; 14-41mg/kg Zn; 0.42mg/kg Pb from soil when irrigated with DWW for 80 days and the concentration in well water irrigation was 28-38 mgL-1 for Zn; 3-7 mgL-1 for Cu and 5-6 mgL-1 for Pb (Siebe, 1995). The concentration of Pb in sewage irrigated soil in Tianjin China’s was reported as 2646.1 mg/kg for Zn; 836.4 mg/kg for Cu; 460.7 mg/kg for Pb and 30.29±3.66 mg/kg Pb was reported in Wheat; 23.46 mgKg-1 Zn and 39.02 mgKg-1 Cu in tomatoes (Weiqing et al., 2016). Comparatively Salvania species accumulated higher concentration of metals from media with higher doses.
The biomass of plants irrigated with DWW increased with time due to the presence of nutrients in wastewater. The heavy metals concentration in plants shows the potentiality to pollute the soils irrigated with domestic wastewater. Both plants showed promising results for the accumulation of metals from wastewater in Constructed wetland. Constructed wetland provided a suitable substratum for the growth of plants. Both plants showed accumulation potentiality and on that behalf could be used as a suitable Ecotechnological tool for ecological restoration and could be implemented in those farmlands which have been irrigated with wastewater for a long time in order to achieve the safe limit. Further research is needed to discover the actual mechanism of plants employed by those plants to scavenge the metal contaminants.
We are highly thankful to Pollution Ecology Laboratory in School of Studies in Botany Jiwaji University Gwalior and Advanced Environmental Testing and Research Lab. Pvt, Ltd Gwalior for providing laboratory and instrumental facilities.