

Research Article - International Research Journal of Biotechnology ( 2023) Volume 14, Issue 2
Received: 23-Mar-2023, Manuscript No. irjob-23-92532; Editor assigned: 25-Mar-2023, Pre QC No. irjob-23-92532(PQ); Reviewed: 08-Apr-2023, QC No. irjob-23-92532; Revised: 13-Apr-2023, Manuscript No. irjob-23-92532(R); Published: 20-Apr-2023, DOI: 10.14303/2141-5153.2023.38
Objectives: The study aimed at determining prevalence and risk factors for glycemic control among type 2 diabetes mellitus patients attending the four health centres in Mbarara City.
Major research question: What is the prevalence of poor glycemic control among type 2 diabetes mellitus patients attending the four health centers in Mbarara city?
Setting: The study was carried out in four health centers at their outpatient clinics in Mbarara city.
Participants: The study enrolled 140 participants who all participated until its completion of which 46(32.9%) were males and 94(67.1%) were females. Consenting adult type 2 diabetes mellitus patients of either sex receiving health care from the targeted study sites were included in the study. Only those who were critically ill or pregnant were excluded.
Results: The overall prevalence of poor glycemic control among T2DM patients attending selected health centers was 80%. Our study enrolled 46(32.9%) males and 94(67.1%) females. 87% of males and 76.6% of females had poor glycemic control. Bivariate and multivariate statistical analyses were done. Significantly associated risk factors with poor glycemic control (Odds Ratio >1) were alcohol intake OR=1.292 (95%CI; 1.175-1.420, P=0.034), random blood sugar OR=2.500 (95%CI; 1.072-5.830, P = 0.031) and T2DM treatment duration OR=2.826 (95%CI; 0.620-12.887, P= 0.002). There was a positive correlation between HbA1c levels and antihyperglycemic therapy, however this relationship was insignificant (r= 0.097, P= 0.480).
Conclusion: Eight in every ten T2DM patients have poor glycemic control. Alcohol intake, long-term duration of T2DM treatment and hyperglycemia impact negatively on a patient’s glycemic control. Health strategies should devote more attention to alleviating the poor glycemic control among T2DM patients in impoverished communities.
There is a significant relationship between poor glycaemic control and the risk of developing type 2 diabetes mellitus associated complications.
WHAT THIS STUDY ADDS?
We report about some of the risk factors that aggravate the type 2 diabetes mellitus glycaemic control problem among patients living in impoverished communities.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY?
Health strategies should devote more attention to alleviating the poor glycemic control among T2DM patients in poor communities.
Diabetes Mellitus (DM) is a serious metabolic disease condition that occurs due to defects in insulin secretion, insulin action or both (Gebreyohannes EA et al., 2019) (American Diabetes A 2019). Global estimates indicate that about 463 million adults (8%) aged 20 to 79 years are living with diabetes and by 2045 these numbers will rise to 700 million (American Diabetes A 2019). Of those living with diabetes, 79% come from low and middle income countries including Uganda (Oluma A et al., 2021) (Saeedi P et al., 2019). In developing countries, 75% of diabetic patients are 45 years old and above (American Diabetes A 2019). A study conducted 10 years ago indicates that Uganda had the fastest growing rate of diabetes mellitus with an estimated 98,000 patients in 2000 to about 1.5 million in 2010 from a population of 30 million people (Nyanzi R et al., 2014).
Type 2 Diabetes Mellitus (T2DM) is the most common form of diabetes mellitus reported; accounting for more than 90% of cases (Barkai L et al., 2020). The global rise in T2DM is occurring fastest in developing countries (Mutebi E et al., 2012). A report showed that the prevalence of diabetes mellitus in the Africa Region ranges between 9.7-15.4% and type 2 diabetes mellitus which comprises almost 90% burden of the disease while the remaining 10% is contributed by other forms of diabetes mellitus (Omar SM et al., 2018). In a related study conducted in Kanungu district in Uganda, the researchers observed a high prevalence of type 2 diabetes patients compared to what other studies had obtained in the region from previous years (Asiimwe D et al., 2020).
Glycemic control were defined as Fasting Blood Sugar (FBS) level of 80 -130 mg/dL (4.4-7.2 mmol/L) or haemoglobin A1C (HbA1c)<7% in adults who are not pregnant (Care D 2019). These two biochemical tests are the focus for efficient type 2 diabetes mellitus management (Ketema EB et al., 2015). Poor glycemic control is a determinant of diabetesrelated complications, thus to prevent or delay the onset of associated complications, patient glucose levels should periodically be monitored (Onodugo OD et al., 2019). The percentage of patients whose blood glucose levels are not well controlled remains high (Mamo Y et al., 2019). Poor glycemic control is a common risk factor for macrovascular complications like peripheral arterial disease, stroke and coronary artery disease, amputations, and microvascular complications like retinopathy, neuropathy and nephropathy (Gebreyohannes EA et al., 2019) (Blair M 2016). A study was conducted at the outpatient diabetes clinic in Mbarara Regional Referral Hospital, the prevalence of poor glycemic control was found to be high (Patrick NB et al., 2021).
In this study, we noted that the selected health centers received a substantial number of diabetic patients; however, there was limited data on glycemic control among the type 2 diabetic patients receiving health care from these facilities. We therefore purposed to determine the magnitude of glycemic control, its risk factors among the T2DM patients in Mbarara, Uganda.
Study area
We conducted this study at four health centers in Mbarara, south-western Uganda. These included; Ruharo Mission Hospital, Kakoba Health Centre III, Mbarara Municipal Council Health Centre IV and Nyamitanga Health Centre III. Ruharo Mission Hospital is a private not-for--forprofit institution. The facility is owned and managed by the church of Uganda under Ankole Diocese, Kamukuzi Division in Mbarara district. It is approximately 4 km west of Mbarara Regional Referral Hospital along Mbarara- Ishaka Road. It had an inpatient bed capacity of 100 and provides specialized health services. Kakoba health Centre III is a government health facility located in Kakoba division, Mbarara Municipality. It is approximately 3.8 km from Mbarara Regional Referral Hospital via Kabale-Mbarara Road and Buremba road. Mbarara Municipal Council Health Centre IV is a government health facility located in Kamukuzi division, Mbarara municipality. It is 1.8 km from Mbarara Regional Referral Hospital via Kabale-Mbarara road. Nyamitanga Health Centre III is a relatively small government health facility located in Karugangama cell, Katete ward, Nyamitanga division in Mbarara municipality. It had four departments, i.e., Outpatients Department (OPD), Store, Antenatal care, and laboratory room.
Study design, population and sample size estimation
A cross-sectional survey was conducted on consented patients who had a known type 2 diabetes mellitus (T2DM) diagnosis and were receiving health care for the treatment and management of the condition from four health centers.
The sample size was calculated using the formula (by Kish and Leslie) below;
Where;
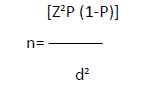
n = sample size,
P = assumed prevalence of diabetes which was 10.1% (Tino S et al., 2019),
(1-P) = probability of not having diabetes,
Z = 1.96 at a standard confidence interval of 95%,
D = 0.05, which was the permissible error term.
Consenting adult type 2 diabetes mellitus patients of either sex receiving health care from the targeted study sites were included in the study. Only those who were critically ill or pregnant were excluded.
Patient and Public involvement
No patient involved.
Ethical Considerations
Approval to conduct this study was granted by the Faculty of Medicine Research Committee (FRC) at Mbarara University of Science and Technology after a rigorous peer review of the study’s protocol. Approval reference number was MUST/MLS/030. Permission to access and engage with the study participants was obtained from the selected study site’s respective in-charges and the Mbarara district town clerk. Written Informed consent was sought from potential participants. The participants who met the inclusion criteria were enrolled into the study by consecutive sampling until the desired sample size was achieved. They were assigned unique identification numbers to observe the privacy and confidentiality of their information.
Data collection
Demographic data was collected using interviewer-guided questionnaires. Blood samples were collected by capillary action and venous blood of 4 millilitres in Ethylene Diamine Tetraacetic Acid (EDTA) coated vacutainers for the assessment of Random Blood Sugar (RBS) and glycated haemoglobin (HbA1c) respectively. RBS was measured with a glucose meter manufactured by GlucoDr.S. A result displayed as ≥ 7.2mmol/l was indicative of Hyperglycaemia. HbA1c was measured on a FinecareTM FIA (Fluorescence Immunoassay) system following standard operating procedures. Participants with HbA1c levels >7% were considered to have a poor glycemic control, while those with HbA1c levels<7% were considered to have a good glycemic control. Body Mass Index (BMI) was also calculated after acquiring the participant’s weight and height. Weight in kilograms (kg) was measured with a calibrated weighing scale, while height in meters (m) was measured with a tape measured in an upright position. Systolic and diastolic blood pressure were measured in millimetre of mercury (mmHg) using an automatic blood pressure machine with the participants in a sitting position.
Statistical Analysis
Data for assessing risk factors and sociodemographic factors was entered into the spread sheets of Microsoft Excel. The data file was then exported into Statistical Package for Social Sciences (SPSS) Version 23 for analysis. Bivariate and multivariate analysis of risk factors was done with the level of significance set at a p-value of < 0.05. The Spearman’s coefficient test was used to determine the correlation between HbA1c levels and anti-hyperglycemic therapy.
Sociodemographic characteristics of participants
A total of 140 participants were enrolled into the study; 46(32.9%) males and 94(67.1%) females. The mean age was 51.53(SD±14.92, median 52) years. Most of the patients were in the age groups of 56 years (51, 36.43%) and 45 to 55 years (49, 35%) with the two age groups accounting for a total of 71.4% of the total population (Table 1).
Prevalence of glycemic control
Prevalence of poor glycemic control among Type 2 Diabetes Mellitus (T2DM) patients was (112: 80%). Glycemic control was reported as good when the HbA1c levels were below 7% and poor when above 7%. Females contributed the highest percentage of study participants 64.3%. Poor glycemic control was found in 71.4% of the participants who were above 45years, had no formal education 39(27.86%), and had attained at least a primary level of education (Table 1). Nyamitanga health centre III had the highest percentage of poor glycemic control (89.47%) and Kakoba health centre III had the lowest at 66.67%.
Variable |
Frequency N (%) | Glycemic Control (HBA1c Levels), N (%) | p-Value | |
|---|---|---|---|---|
| Good | Poor | |||
| Sex | 0.152 | |||
| Male | 46(32.9) | 6(13) | 40(87) | |
| Female | 94(67.1) | 22(23.4) | 72(76.6) | |
| Age In Years | 0.436 | |||
| 23-33 | 24(17.14) | 06(25) | 18(75) | |
| 34-44 | 16(11.43) | 02(12.5) | 14(87.5) | |
| 45-55 | 49(35) | 10(20.41) | 39(79.59) | |
| ≥56 | 51(36.43) | 10(19.61) | 41(80.39) | |
| Education Level | 0.223 | |||
| No Formal Education | 39(27.86) | 5(12.8) | 34(87.2) | 34(87.2) |
| Primary | 56(40) | 14(25) | 42(75) | |
| Secondary | 25(17.86) | 1(4) | 24(96) | |
| Tertiary | 20(14.28) | 8(40) | 12(60) | |
Risk factors for glycemic control
Bivariate analysis showed that random blood sugar, alcohol intake, day activity, duration of T2DM treatment, and carbohydrate intake were significantly related to poor glycemic control (Table 2).
Risk Factor |
Frequency | Glycemic Control (Hba1c Levels) | P-Value | |
|---|---|---|---|---|
| Good (Hba1c<7%) | Poor (Hba1c>7%) | |||
| BMI (Kg/M2) | 0.908 | |||
| Underweight | 2(1.43) | 0(0) | 2(100) | |
| Healthy Weight | 40(28.57) | 9(22.5) | 31(77.5) | |
| Overweight | 58(41.43) | 11(19.0) | 47(81) | |
| Obese | 40(28.57) | 8(20) | 32(80) | |
| Blood Pressure (Mmhg) | 0.060 | |||
| Hypertensive | 50(35.71) | 12(24) | 38(76) | |
| Prehypertensive | 65(46.43) | 13(20) | 52(80) | |
| Normal | 25(17.86) | 3(12) | 22(88) | |
| RBS (Mmol/L) | 0.031* | |||
| Normal | 46(32.9) | 14(30.4) | 32(69.9) | |
| High | 97(67.1) | 14(14.9) | 80(85.1) | |
| Smoking | 0.619 | |||
| Yes | 1(100) | 0(0) | 1(100) | |
| No | 139(99.3) | 28(20.1) | 111(79.9) | |
| Alcohol Intake | 0.034* | |||
| Yes | 16(11.4) | 0(0) | 16(14.3) | |
| No | 124(88.6) | 28(22.6) | 96(77.4) | |
| Medication Used | 0.48 | |||
| Metformin | 21(15) | 5(23.8) | 16(76.2) | |
| Insulin Therapy | 10(7.14) | 1(10) | 9(90) | |
| Metformin And Sulphonylureas | 87(62.14) | 20(23) | 67(77) | |
| Metformin And Insulin Therapy | 19(13.57) | 1(5.3) | 18(94.7) | |
| Herbal Medicine | 1(0.71) | 0(0) | 1(100) | |
| None | 2(1.43) | 1(50) | 1(50) | |
| Day Activities | 0.037* | |||
| Sitting | 41(28.29) | 5(12.2) | 36(87.8) | |
| Standing | 62(44.29) | 12(19.4) | 50(80.6) | |
| Walking | 15(10.71) | 2(13.3) | 13(86.7) | |
| Sitting And Walking | 1(0.71) | 1(100) | 0(0) | |
| Standing And Walking | 17(12.14) | 7(41.2) | 10(58.8) | |
| All Activities | 4(2.86) | 1(25) | 3(75) | |
| Exercise | 0.82 | |||
| Daily | 13(9.29) | 1(7.7) | 12(92.3) | |
| Sometimes | 107(76.43) | 24(22.4) | 83(77.6) | |
| Never | 20(14.29) | 3(15) | 17(85) | |
| Underlying Health Condition | 0.734 | |||
| Asthma | 1(0.71) | 1(100) | 0(0) | |
| HIV/AIDS | 3(2.14) | 0(0) | 3(100) | |
| Allergies | 1(0.07) | 0(0) | 1(100) | |
| High Blood Pressure | 66(47.14) | 12(18.2) | 54(81.8) | |
| Low Blood Pressure | 1(0.071) | 1(100) | 0(0) | |
| None | 68(48.57) | 14(20.6) | 54(79.4) | |
| T2DM Treatment Duration | 0.001* | |||
| Below 7 Years | 116(82.9) | 28(24.1) | 88(75.9) | |
| Above 8 Years | 24(17.1) | 2(8.3) | 24(17.1) | |
| Carbohydrates Main Meal | 0.041* | |||
| Yes | 137(97.9) | 26(19) | 111(81) | |
| No | 3(2.1) | 2(66.7) | 1(33.3) | |
| Diet Knowledge | 0.575 | |||
| Yes | 39(27.86) | 9(23.1) | 30(76.9) | |
| No | 101(72.14) | 19(18.8) | 82(81.2) | |
| Level of Significance Set At P<0.05, HBA1c- Glycated Haemoglobin, RBS-Random Blood Sugar,T2DM-Type 2 Diabetes Mellitus, BMI-Body Mass Index | ||||
Using binary regression to carry out a multivariate analysis, alcohol intake, random blood sugar, day activity, carbohydrate intake, and T2DM treatment duration were significant risk factors for poor glycemic control (P<0.05). The odds ratio further shows that alcohol intake, random blood sugar (RBS) levels, and T2DM treatment duration are significantly associated with poor glycemic control (OR>1). Risk factors including day activity and carbohydrate main intake had no association with poor glycemic control (OR<1), Table 3.
Variables |
Odds Ratio (95% CI) | p- value |
|---|---|---|
| Alcohol Intake | 1.292(1.175-1.420) | 0.034 |
| RBS | 2.500(1.072-5.830) | 0.031 |
| Day Activities | 0.459(0.161-1.305) | 0.038 |
| Carbohydrates Main Intake | 0.117(0.010-1.341) | 0.044 |
| T2DM Treatment Duration | 2.826(0.620-12.887) | 0.002 |
| Level of significance set at P<0.05, RBS-Random Blood Sugar, T2DM-Type 2 Diabetes Mellitus, CI- Confidence Interval | ||
Correlation between HbA1c levels and antihyperglycemic therapy
There was a positive correlation between HbA1c levels and anti-hyperglycemic therapy used by the participants, although it was negligible (rho=0.097, P=0.480). This correlation was not significant in the study.
Prevalence of glycemic control
Our study enrolled 140 participants and most of them (112, 80%) had poor glycemic control (HbA1c > 7%). The prevalence of poor glycemic control was high at 80% which is slightly similar to other studies done in Uganda, 84.3% (Patrick NB et al., 2021) and Kenya, 81.9% (Nduati NJ et al., 2016). However , this prevalence (80%) is higher than different rates of poor glycemic control which were reported in other similar studies, for example , (73.3%, 183) in Uganda (Kibirige D et al., 2014), (71.9%,243) in Eastern Sudan (Omar SM et al., 2018), (64.1%, 271) in Ethiopia (Oluma A et al., 2021), (49.8%, 114) in Tanzania (Gunda DW et al., 2020) , (75.2%,79) in Senegal (BeLue R et al., 2016) and (62%,74) in Nigeria (Ngwogu K et al., 2012). The prevalence discrepancy is likely due to the different social economical differences in these populations, the nature of the studies that were conducted, assessment of the glycated haemoglobin test (HbA1c) and lifestyle behaviours such as excessive alcohol intake that may hinder attainment of a controlled glycemic status.
Risk factors associated with poor glycemic control
Alcohol consumption influences diabetes evolution in such a way that it can interfere with self-care behavior, which is an important determinant of type 2 diabetes mellitus disease prognosis (Salama MS et al., 2021). Our study has indicated that alcohol consumption is a potential risk factor for poor glycemic control. It is important to note that this was a selfreported variable. Moreover, it was interesting to find that a proportion of those who had reported to not consume alcohol actually had a poor glycemic control as well. This implies that there could be more than what meets the eye regarding attaining good glycemic control among type 2 diabetes patients.
We also found that participants who were categorized as either pre-hypertensive and hypertensive (with raised blood pressure) had poor glycemic control. It was the commonest health complication presented by most of our participants which agrees with the findings of a study done in Uganda (Kibirige D et al., 2014) whose results also indicated that hypertension was the most frequently screened complication related to type 2 diabetes mellitus. Other several studies have shown that the majority of patients with diabetes also end up developing hypertension (Emeka PM et al., 2017). High blood pressure was, however, not significantly associated with glycemic control as depicted in our results analysis.
We observed a positive relationship between the duration of type 2 diabetes mellitus and glycaemic control. This agrees with studies conducted in Ethiopia and China in which participants who had a diabetes duration of more than 5 years had a poor glycemic control compared to those who had had the disease condition for less than 2 years (Oluma A et al., 2021), (Li J et al., 2018). This could be due to the fact that cell function usually worsens as the duration of diabetes increases from the time of diagnosis through follow-up. Non-adherence to medication and failure adjust to an appropriate life style after the initial type 2 diabetes mellitus diagnosis makes it difficult for treatment to be effective.
Most of the study participants had carbohydrates as their main meal. Carbohydrates form a big part of a typical diet in most Ugandan communities. Increased consumption of carbohydrates was shown to have a high glycemic index and associated with poor glycemic control among type 2 diabetes mellitus patients (Bonsembiante L et al., 2021). Since most of the participants took carbohydrates as a main meal, this could as well possibly explain the increase in random blood sugar levels with a consistent hyperglycemic state likely to impact negatively on the HbA1c levels.
Day time activities were linked to good glycemic control among our study participants. A proportion of them concurred with engaging in either standing or walking drills. Exercise which goes hand in hand with day activities was not significant in our study. The good glycemic control among those who were standing/walking is supported by the beneficial effect of physical activity such as improvement of glucose tolerance, insulin sensitivity, and reduction in HbA1c levels (Cannata F et al., 2020). The mean RBS was 10.9mmol/l, which corresponds to HbA1c level of approximately 9.0% and this is above the upper reference limit of 7.0% indicating a correlation between HbA1c and blood sugar levels of which all are indicative of poor glycemic control.
Body Mass Index (BMI), age in years, and education level did not significantly affect glycemic control. These findings are in agreement with a report from Eastern Sudan where BMI, aging, and education level were not associated with poor glycemic control (Omar SM et al., 2019). In another study, it was demonstrated that age-associated decline in mitochondrial function contributes to insulin resistance in the elderly (Petersen KF et al., 2003). Educational programs that emphasize adherence to treatment regimens as a whole, especially to diet, to exercise, and to regular follow-up are of greater benefit in glycemic control compared to compliance of medications (Al-Rasheedi AAS 2014). Such programs help the illiterate to understand the factors that affect their glycemic control. Body mass index has a strong relationship with type 2 diabetes mellitus and insulin resistance, a finding we couldn’t ascertain. In obese individuals, the amount of non-esterified fatty acids, glycerol, hormones, cytokines, proinflammatory markers, and other substances that are involved in the development of insulin resistance is usually increased (Kahn SE et al., 2006).
Correlation between HbA1c levels and anti-hyperglycemic therapy
The study results revealed that the use of oral antihyperglycemic therapy (metformin +sulfonylureas) showed a better glycemic control compared to those who used insulin and a combination of (insulin + metformin). This finding agrees with other studies conducted in Ethiopia, China and Tanzania (Mamo Y et al., 2019), (Dong Q et al., 2019), (Kamuhabwa AR et al., 2014). However, we believe that the difference in glycemic control could have been due to long-term duration of type 2 diabetes mellitus and a likely occurrence of diabetic complications such as high blood pressure among those who took insulin. We also hypothesize that non-adherence to the combination therapy as a result of the costs involved in obtaining insulin, a rare drug in health centers, is culpable for poor glycemic control. However, the association between anti-hyperglycemic therapy and glycemic control was not significant in our study.
Study limitation
Since we collected data from outpatient departments of health care facilities without established diabetic clinics, some of the variables included self-reported data such as the date of diabetes diagnosis and thus could not be verified easily.
Eight in every ten type 2 diabetes mellitus patients at the selected health centers have poor glycemic control. Risk Factors such as alcohol intake, long-term duration of type 2 diabetes mellitus treatment, and hyperglycemia have a negative impact on a patient’s glycemic control. Clinical and public health strategies should devote more attention to alleviating the poor glycemic control among type 2 diabetes mellitus patients in impoverished communities.
Daphine Nabukenya, Davis Nduhuura, Derrick Turyasingura, Lillian Racheal Sabano and Bernard Kutuusa made substantial contributions to the conception and design of the work. They also carried out the data collection, its analysis and interpretation. Joseph Ndarubweine and Ritah Kiconco contributed to the design of the work and critically revised the manuscript for important intellectual content. All authors read and approved the work before submission.
The data used to support the findings of this study are available from the corresponding author upon request.
We declare that there is no conflict of interest regarding the publication of this work.
This research study was self-sponsored.
We express our gratitude to the administrators of the various study sites for granting us permission and a conducive environment to carry out this study.
There are no supplementary files accompanying this manuscript.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref